Ångstrom-scale silver particle-embedded carbomer gel promotes wound healing by inhibiting bacterial colonization and inflammation
- PMID: 33097529
- PMCID: PMC7608828
- DOI: 10.1126/sciadv.aba0942
Ångstrom-scale silver particle-embedded carbomer gel promotes wound healing by inhibiting bacterial colonization and inflammation
Abstract
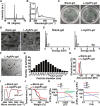
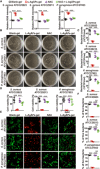
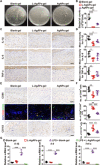
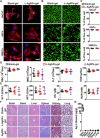
Poor wound healing after diabetes or extensive burn remains a challenging problem. Recently, we presented a physical approach to fabricate ultrasmall silver particles from Ångstrom scale to nanoscale and determined the antitumor efficacy of Ångstrom-scale silver particles (AgÅPs) in the smallest size range. Here we used the medium-sized AgÅPs (65.9 ± 31.6 Å) to prepare carbomer gel incorporated with these larger AgÅPs (L-AgÅPs-gel) and demonstrated the potent broad-spectrum antibacterial activity of L-AgÅPs-gel without obvious toxicity on wound healing-related cells. Induction of reactive oxygen species contributed to L-AgÅPs-gel-induced bacterial death. Topical application of L-AgÅPs-gel to mouse skin triggered much stronger effects than the commercial silver nanoparticles (AgNPs)-gel to prevent bacterial colonization, reduce inflammation, and accelerate diabetic and burn wound healing. L-AgÅPs were distributed locally in skin without inducing systemic toxicities. This study suggests that L-AgÅPs-gel represents an effective and safe antibacterial and anti-inflammatory material for wound therapy.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- Dainichi T., Kitoh A., Otsuka A., Nakajima S., Nomura T., Kaplan D. H., Kabashima K., The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat. Immunol. 19, 1286–1298 (2018). - PubMed
-
- Kalan L. R., Brennan M. B., The role of the microbiome in nonhealing diabetic wounds. Ann. N. Y. Acad. Sci. 1435, 79–92 (2019). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

