Itaconate controls the severity of pulmonary fibrosis
- PMID: 33097591
- PMCID: PMC7116646
- DOI: 10.1126/sciimmunol.abc1884
Itaconate controls the severity of pulmonary fibrosis
Abstract
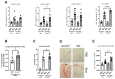
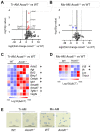
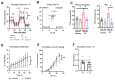
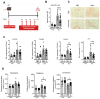
Idiopathic pulmonary fibrosis (IPF) is a fatal lung disease in which airway macrophages (AMs) play a key role. Itaconate has emerged as a mediator of macrophage function, but its role during fibrosis is unknown. Here, we reveal that itaconate is an endogenous antifibrotic factor in the lung. Itaconate levels are reduced in bronchoalveolar lavage, and itaconate-synthesizing cis-aconitate decarboxylase expression (ACOD1) is reduced in AMs from patients with IPF compared with controls. In the murine bleomycin model of pulmonary fibrosis, Acod1−/− mice develop persistent fibrosis, unlike wild-type (WT) littermates. Profibrotic gene expression is increased in Acod1−/− tissue-resident AMs compared with WT, and adoptive transfer of WT monocyte-recruited AMs rescued mice from disease phenotype. Culture of lung fibroblasts with itaconate decreased proliferation and wound healing capacity, and inhaled itaconate was protective in mice in vivo. Collectively, these data identify itaconate as critical for controlling the severity of lung fibrosis, and targeting this pathway may be a viable therapeutic strategy.
Conflict of interest statement
Unrelated to the current work, TMM has, via his institution, received industry-academic funding from GlaxoSmithKline R&D and UCB and has received consultancy or speakers fees from Apellis, Astra Zeneca, Bayer, Blade Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Galapagos, GlaxoSmithKline R&D, Indalo, Novartis, Pliant, ProMetic, Respivnat, Roche, Samumed and UCB. PLM received, unrelated to the submitted work, speaker and advisory board fees from Boehringer Ingelheim and Hoffmann-La Roche, via his institution. The authors declare no further conflicts of interest.
Figures



References
-
- Allden SJ, Ogger PP, Ghai P, McErlean P, Hewitt R, Toshner R, Walker SA, Saunders P, Kingston S, Molyneaux PL, et al. The Transferrin Receptor CD71 Delineates Functionally Distinct Airway Macrophage Subsets during Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2019;200:209–219. - PMC - PubMed
-
- Basler T, Jeckstadt S, Valentin-Weigand P, Goethe R. Mycobacterium paratuberculosis, Mycobacterium smegmatis and lipopolysaccharide induce different transcriptional and post-transcriptional regulation of the IRG1 gene in murine macrophages. J Leukoc Biol. 2006;79:628–638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

