Dissociation of Medial Frontal β-Bursts and Executive Control
- PMID: 33097634
- PMCID: PMC7687065
- DOI: 10.1523/JNEUROSCI.2072-20.2020
Dissociation of Medial Frontal β-Bursts and Executive Control
Abstract
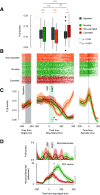
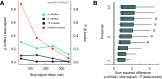
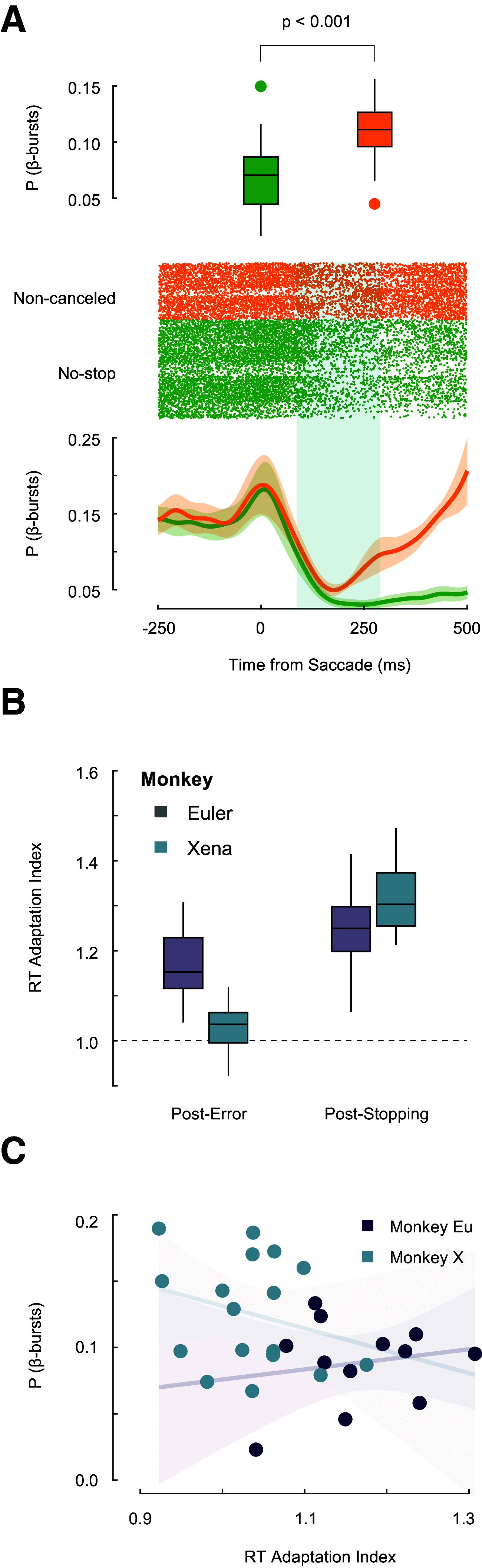
The neural mechanisms of executive and motor control concern both basic researchers and clinicians. In human studies, preparation and cancellation of movements are accompanied by changes in the β-frequency band (15-29 Hz) of electroencephalogram (EEG). Previous studies with human participants performing stop signal (countermanding) tasks have described reduced frequency of transient β-bursts over sensorimotor cortical areas before movement initiation and increased β-bursting over medial frontal areas with movement cancellation. This modulation has been interpreted as contributing to the trial-by-trial control of behavior. We performed identical analyses of EEG recorded over the frontal lobe of macaque monkeys (one male, one female) performing a saccade countermanding task. While we replicate the occurrence and modulation of β-bursts associated with initiation and cancellation of saccades, we found that β-bursts occur too infrequently to account for the observed stopping behavior. We also found β-bursts were more common after errors, but their incidence was unrelated to response time (RT) adaptation. These results demonstrate the homology of this EEG signature between humans and macaques but raise questions about the current interpretation of β band functional significance.SIGNIFICANCE STATEMENT The finding of increased β-bursting over medial frontal cortex with movement cancellation in humans is difficult to reconcile with the finding of modulation too late to contribute to movement cancellation in medial frontal cortex of macaque monkeys. To obtain comparable measurement scales, we recorded electroencephalogram (EEG) over medial frontal cortex of macaques performing a stop signal (countermanding) task. We replicated the occurrence and modulation of β-bursts associated with the cancellation of movements, but we found that β-bursts occur too infrequently to account for observed stopping behavior. Unfortunately, this finding raises doubts whether β-bursts can be a causal mechanism of response inhibition, which impacts future applications in devices such as brain-machine interfaces.
Keywords: EEG; countermanding; error monitoring; response inhibition; stop signal; stopping.
Copyright © 2020 the authors.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
