Skin swabbing is a refined technique to collect DNA from model fish species
- PMID: 33097784
- PMCID: PMC7584585
- DOI: 10.1038/s41598-020-75304-1
Skin swabbing is a refined technique to collect DNA from model fish species
Abstract
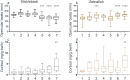
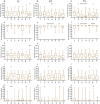
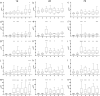
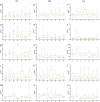
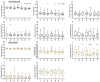
Model fish species such as sticklebacks and zebrafish are frequently used in studies that require DNA to be collected from live animals. This is typically achieved by fin clipping, a procedure that is simple and reliable to perform but that can harm fish. An alternative procedure to sample DNA involves swabbing the skin to collect mucus and epithelial cells. Although swabbing appears to be less invasive than fin clipping, it still requires fish to be netted, held in air and handled-procedures that can cause stress. In this study we combine behavioural and physiological analyses to investigate changes in gene expression, behaviour and welfare after fin clipping and swabbing. Swabbing led to a smaller change in cortisol release and behaviour on the first day of analysis compared to fin clipping. It also led to less variability in data suggesting that fewer animals need to be measured after using this technique. However, swabbing triggered some longer term changes in zebrafish behaviour suggesting a delayed response to sample collection. Skin swabbing does not require the use of anaesthetics and triggers fewer changes in behaviour and physiology than fin clipping. It is therefore a more refined technique for DNA collection with the potential to improve fish health and welfare.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

