PRL-2 phosphatase is required for vascular morphogenesis and angiogenic signaling
- PMID: 33097786
- PMCID: PMC7584612
- DOI: 10.1038/s42003-020-01343-z
PRL-2 phosphatase is required for vascular morphogenesis and angiogenic signaling
Abstract
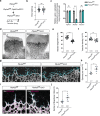
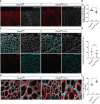
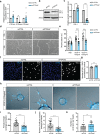
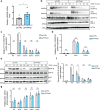
Protein tyrosine phosphatases are essential modulators of angiogenesis and have been identified as novel therapeutic targets in cancer and anti-angiogenesis. The roles of atypical Phosphatase of Regenerative Liver (PRL) phosphatases in this context remain poorly understood. Here, we investigate the biological function of PRL phosphatases in developmental angiogenesis in the postnatal mouse retina and in cell culture. We show that endothelial cells in the retina express PRL-2 encoded by the Ptp4a2 gene, and that inducible endothelial and global Ptp4a2 mutant mice exhibit defective retinal vascular outgrowth, arteriovenous differentiation, and sprouting angiogenesis. Mechanistically, PTP4A2 deletion limits angiogenesis by inhibiting endothelial cell migration and the VEGF-A, DLL-4/NOTCH-1 signaling pathway. This study reveals the importance of PRL-2 as a modulator of vascular development.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

