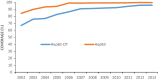
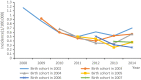
Protective effect of vaccinating infants with a 5 µg recombinant yeast-derived hepatitis B vaccine and the need for a booster dose in China
- PMID: 33097788
- PMCID: PMC7584599
- DOI: 10.1038/s41598-020-75338-5
Protective effect of vaccinating infants with a 5 µg recombinant yeast-derived hepatitis B vaccine and the need for a booster dose in China
Abstract
In 2002, China integrated hepatitis B vaccine (HepB) into its Expanded Program on Immunization (EPI) using HepB vaccine containing 5 µg of antigen. Although not recommended nationally, there was a common clinical practice in China of screening children for anti-HBs antibody level and giving a booster dose to HBV surface antigen (HBsAg)-negative children with non-protective anti-HBs antibody levels. We report an evaluation of the protective effectiveness of the 5 µg HepB vaccine and the serological response to the booster dose. We used data from a 2014 hepatitis B serological survey to determine HBsAg positivity and anti-HBs antibody levels among children who received and did not receive a booster dose. We determined HepB coverage from the Children Immunization Information Management System (CIIMS). We obtained and analyzed reports of acute Hepatitis B (AHB) during 2008-2014 obtained from the National Notifiable Disease Reporting System (NNDRS). The HBsAg-positive rate among children who had not received a booster dose was 0.41%, and did not increase with age (i.e., time since infant immunization). The anti-HBs positivity rate among the 6% of children who received a booster dose (88.41%) was higher than among those who had not received a booster (60.85%); anti-HBs antibody levels declined with age regardless of booster dose status. There was no statistically significant difference in HBsAg positivity between children who received a booster dose and those who did not. The AHB incidence among children born between 2002 and 2007 did not increase with age. Use of routine 5 µg HepB vaccine was not associated with an increase in AHB or of HBsAg positivity by time since vaccination, providing supportive evidence that individuals vaccinated with the 5 µg HepB vaccine do not need a booster dose. Although a booster dose was associated with increases in anti-HBs antibody levels, our study provided no evidence to support the need for this clinical practice. We should continue to strengthen serological monitoring of children, especially for those born to HBsAg positive mothers.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Post-vaccination serologic testing of infants born to hepatitis B surface antigen positive mothers in 4 provinces of China.Vaccine. 2017 Jul 24;35(33):4229-4235. doi: 10.1016/j.vaccine.2017.06.019. Epub 2017 Jun 23. Vaccine. 2017. PMID: 28651839
-
The effects of different dosage levels of hepatitis B vaccine as booster on anti-HBs-negative children 5-15 y after primary immunization; China, 2009-2010.Hum Vaccin Immunother. 2014;10(2):498-504. doi: 10.4161/hv.26936. Epub 2013 Nov 5. Hum Vaccin Immunother. 2014. PMID: 24192508 Free PMC article.
-
The effects of booster vaccination on hepatitis B vaccine in anti-HBs negative infants of HBsAg-positive mothers after primary vaccination.Hum Vaccin Immunother. 2013 Jun;9(6):1292-5. doi: 10.4161/hv.24021. Epub 2013 Feb 19. Hum Vaccin Immunother. 2013. PMID: 23422028 Free PMC article.
-
Risk of early horizontal transmission of hepatitis B virus in children of uninfected mothers in sub-Saharan Africa: a systematic review and meta-analysis.Lancet Glob Health. 2023 May;11(5):e715-e728. doi: 10.1016/S2214-109X(23)00131-6. Lancet Glob Health. 2023. PMID: 37061310
-
A retrospective study of hepatitis B vaccination in preterm birth and low birth weight infants born to hepatitis B surface antigen-positive mothers: Time to close the policy-practice gap.Hum Vaccin Immunother. 2022 Dec 30;18(7):2155390. doi: 10.1080/21645515.2022.2155390. Epub 2022 Dec 14. Hum Vaccin Immunother. 2022. PMID: 36514905 Free PMC article. Review.
Cited by
-
The impact of glucose tolerance state on seropositivity rate after hepatitis B vaccination.Sci Rep. 2022 Feb 23;12(1):3087. doi: 10.1038/s41598-022-07163-x. Sci Rep. 2022. PMID: 35197568 Free PMC article.
-
Immune response to GeneVac-BⓇ (rDNA I.P. hepatitis B vaccine) in vaccinated persons with a standard schedule in Bobo-Dioulasso, Burkina Faso.IJID Reg. 2024 Nov 2;13:100483. doi: 10.1016/j.ijregi.2024.100483. eCollection 2024 Dec. IJID Reg. 2024. PMID: 39639948 Free PMC article.
-
Paradoxical HBsAg and anti-HBs coexistence among Chronic HBV Infections: Causes and Consequences.Int J Biol Sci. 2021 Mar 11;17(4):1125-1137. doi: 10.7150/ijbs.55724. eCollection 2021. Int J Biol Sci. 2021. PMID: 33867835 Free PMC article. Review.
-
Coverage with Timely Administered Vaccination against Hepatitis B Virus and Its Influence on the Prevalence of HBV Infection in the Regions of Different Endemicity.Vaccines (Basel). 2021 Jan 23;9(2):82. doi: 10.3390/vaccines9020082. Vaccines (Basel). 2021. PMID: 33498794 Free PMC article.
-
Increasing Prevalence of Occult HBV Infection in Adults Vaccinated Against Hepatitis B at Birth.Vaccines (Basel). 2025 Feb 12;13(2):174. doi: 10.3390/vaccines13020174. Vaccines (Basel). 2025. PMID: 40006721 Free PMC article.
References
-
- Global hepatitis report, 2017.https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/.
-
- Global health sector strategy on viral hepatitis 2016–2021.https://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/.
-
- Centers for Disease Control and Prevention. Hepatitis B questions and answers for health professionals. 2018 https://www.cdc.gov/hepatitis/hbv/hbvfaq.htm. Accessed 29 October 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical