Direct signaling of TL1A-DR3 on fibroblasts induces intestinal fibrosis in vivo
- PMID: 33097818
- PMCID: PMC7584589
- DOI: 10.1038/s41598-020-75168-5
Direct signaling of TL1A-DR3 on fibroblasts induces intestinal fibrosis in vivo
Abstract
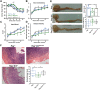
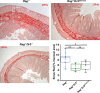
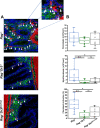
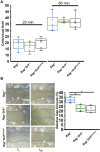
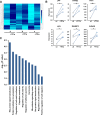
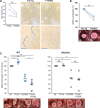
Tumor necrosis factor-like cytokine 1A (TL1A, TNFSF15) is implicated in inflammatory bowel disease, modulating the location and severity of inflammation and fibrosis. TL1A expression is increased in inflamed mucosa and associated with fibrostenosing Crohn's disease. Tl1a-overexpression in mice causes spontaneous ileitis, and exacerbates induced proximal colitis and fibrosis. Intestinal fibroblasts express Death-receptor 3 (DR3; the only know receptor for TL1A) and stimulation with TL1A induces activation in vitro. However, the contribution of direct TL1A-DR3 activation on fibroblasts to fibrosis in vivo remains unknown. TL1A overexpressing naïve T cells were transferred into Rag-/- , Rag-/- mice lacking DR3 in all cell types (Rag-/-Dr3-/-), or Rag-/- mice lacking DR3 only on fibroblasts (Rag-/-Dr3∆Col1a2) to induce colitis and fibrosis, assessed by clinical disease activity index, intestinal inflammation, and collagen deposition. Rag-/- mice developed overt colitis with intestinal fibrostenosis. In contrast, Rag-/-Dr3-/- demonstrated decreased inflammation and fibrosis. Despite similar clinical disease and inflammation as Rag-/-, Rag-/-Dr3∆Col1a2 exhibited reduced intestinal fibrosis and attenuated fibroblast activation and migration. RNA-Sequencing of TL1A-stimulated fibroblasts identified Rho signal transduction as a major pathway activated by TL1A and inhibition of this pathway modulated TL1A-mediated fibroblast functions. Thus, direct TL1A signaling on fibroblasts promotes intestinal fibrosis in vivo. These results provide novel insight into profibrotic pathways mediated by TL1A paralleling its pro-inflammatory effects.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Inflammation-independent TL1A-mediated intestinal fibrosis is dependent on the gut microbiome.Mucosal Immunol. 2018 Sep;11(5):1466-1476. doi: 10.1038/s41385-018-0055-y. Epub 2018 Jul 9. Mucosal Immunol. 2018. PMID: 29988118 Free PMC article.
-
Tumor Necrosis Factor-like Cytokine TL1A and Its Receptors DR3 and DcR3: Important New Factors in Mucosal Homeostasis and Inflammation.Inflamm Bowel Dis. 2015 Oct;21(10):2441-52. doi: 10.1097/MIB.0000000000000492. Inflamm Bowel Dis. 2015. PMID: 26099067 Review.
-
TL1A (TNFSF15) and DR3 (TNFRSF25): A Co-stimulatory System of Cytokines With Diverse Functions in Gut Mucosal Immunity.Front Immunol. 2019 Mar 27;10:583. doi: 10.3389/fimmu.2019.00583. eCollection 2019. Front Immunol. 2019. PMID: 30972074 Free PMC article. Review.
-
Differential Levels of Tl1a Affect the Expansion and Function of Regulatory T Cells in Modulating Murine Colitis.Inflamm Bowel Dis. 2016 Mar;22(3):548-59. doi: 10.1097/MIB.0000000000000653. Inflamm Bowel Dis. 2016. PMID: 26818423 Free PMC article.
-
Inhibition of a novel fibrogenic factor Tl1a reverses established colonic fibrosis.Mucosal Immunol. 2014 Nov;7(6):1492-503. doi: 10.1038/mi.2014.37. Epub 2014 May 21. Mucosal Immunol. 2014. PMID: 24850426 Free PMC article.
Cited by
-
Preventing fibrosis in IBD: update on immune pathways and clinical strategies.Expert Rev Clin Immunol. 2024 Jul;20(7):727-734. doi: 10.1080/1744666X.2024.2330604. Epub 2024 Mar 21. Expert Rev Clin Immunol. 2024. PMID: 38475672 Free PMC article. Review.
-
Research progress on the relationship between Paneth cells-susceptibility genes, intestinal microecology and inflammatory bowel disease.World J Clin Cases. 2023 Dec 6;11(34):8111-8125. doi: 10.12998/wjcc.v11.i34.8111. World J Clin Cases. 2023. PMID: 38130785 Free PMC article. Review.
-
Tackling Inflammatory Bowel Diseases: Targeting Proinflammatory Cytokines and Lymphocyte Homing.Pharmaceuticals (Basel). 2022 Aug 30;15(9):1080. doi: 10.3390/ph15091080. Pharmaceuticals (Basel). 2022. PMID: 36145301 Free PMC article. Review.
-
Inflammation accelerating intestinal fibrosis: from mechanism to clinic.Eur J Med Res. 2024 Jun 18;29(1):335. doi: 10.1186/s40001-024-01932-2. Eur J Med Res. 2024. PMID: 38890719 Free PMC article. Review.
-
Eicosatetraynoic Acid Regulates Profibrotic Pathways in an Induced Pluripotent Stem Cell-Derived Macrophage-Human Intestinal Organoid Model of Crohn's Disease.J Crohns Colitis. 2025 Feb 4;19(2):jjae139. doi: 10.1093/ecco-jcc/jjae139. J Crohns Colitis. 2025. PMID: 39212594
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

