Hepatocyte Nuclear Factor 4α Prevents the Steatosis-to-NASH Progression by Regulating p53 and Bile Acid Signaling (in mice)
- PMID: 33098092
- PMCID: PMC8062586
- DOI: 10.1002/hep.31604
Hepatocyte Nuclear Factor 4α Prevents the Steatosis-to-NASH Progression by Regulating p53 and Bile Acid Signaling (in mice)
Abstract
Background and aims: Hepatocyte nuclear factor 4α (HNF4α) is highly enriched in the liver, but its role in the progression of nonalcoholic liver steatosis (NAFL) to NASH has not been elucidated. In this study, we investigated the effect of gain or loss of HNF4α function on the development and progression of NAFLD in mice.
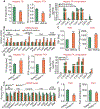
Approach and results: Overexpression of human HNF4α protected against high-fat/cholesterol/fructose (HFCF) diet-induced steatohepatitis, whereas loss of Hnf4α had opposite effects. HNF4α prevented hepatic triglyceride accumulation by promoting hepatic triglyceride lipolysis, fatty acid oxidation, and VLDL secretion. Furthermore, HNF4α suppressed the progression of NAFL to NASH. Overexpression of human HNF4α inhibited HFCF diet-induced steatohepatitis in control mice but not in hepatocyte-specific p53-/- mice. In HFCF diet-fed mice lacking hepatic Hnf4α, recapitulation of hepatic expression of HNF4α targets cholesterol 7α-hydroxylase and sterol 12α-hydroxylase and normalized hepatic triglyceride levels and attenuated steatohepatitis.
Conclusions: The current study indicates that HNF4α protects against diet-induced development and progression of NAFLD by coordinating the regulation of lipolytic, p53, and bile acid signaling pathways. Targeting hepatic HNF4α may be useful for treatment of NASH.
© 2020 by the American Association for the Study of Liver Diseases.
Figures


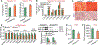




References
-
- Edmison J, McCullough AJ. Pathogenesis of non-alcoholic steatohepatitis: human data. Clin Liver Dis 2007;11:75–104, ix. - PubMed
-
- Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology 1998;114:842–845. - PubMed
-
- Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis 2008;28:370–379. - PubMed
-
- Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci 2010;55:560–578. - PubMed
-
- Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113–121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

