Phase II Trial of Neoadjuvant Carboplatin and Nab-Paclitaxel in Patients with Triple-Negative Breast Cancer
- PMID: 33098195
- PMCID: PMC7930424
- DOI: 10.1002/onco.13574
Phase II Trial of Neoadjuvant Carboplatin and Nab-Paclitaxel in Patients with Triple-Negative Breast Cancer
Abstract
Background: In this phase II clinical trial, we evaluated the efficacy of the nonanthracycline combination of carboplatin and nab-paclitaxel in early stage triple-negative breast cancer (TNBC).
Patients and methods: Patients with newly diagnosed stage II-III TNBC (n = 69) were treated with neoadjuvant carboplatin (area under the curve 6) every 28 days for four cycles plus nab-paclitaxel (100 mg/m2 ) weekly for 16 weeks. Pathological complete response (pCR) and residual cancer burden (RCB) were analyzed with germline mutation status, tumor-infiltrating lymphocytes (TILs), TNBC molecular subtype, and GeparSixto immune signature (GSIS).
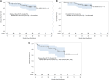
Results: Sixty-seven patients were evaluable for safety and response. Fifty-three (79%) patients experienced grade 3/4 adverse events, including grade 3 anemia (43%), neutropenia (39%), leukopenia (15%), thrombocytopenia (12%), fatigue (7%), peripheral neuropathy (7%), neutropenia (16%), and leukopenia (1%). Twenty-four patients (35%) had at least one dose delay, and 50 patients (72%) required dose reduction. Sixty-three (94%) patients completed scheduled treatment. The responses were as follows: 32 of 67 patients (48%) had pCR (RCB 0), 10 of 67 (15%) had RCB I, 19 of 67 (28%) had RCB II, 5 of 67 (7%) had RCB III, and 1 of 67 (2%) progressed and had no surgery. Univariate analysis showed that immune-hot GSIS and DNA repair defect (DRD) were associated with higher pCR with odds ratios of 4.62 (p = .005) and 4.76 (p = .03), respectively, and with RCB 0/I versus RCB II/III with odds ratio 4.80 (p = .01). Immune-hot GSIS was highly correlated with DRD status (p = .03), TIL level (p < .001), and TNBC molecular subtype (p < .001). After adjusting for age, race, stage, and grade, GSIS remained associated with higher pCR and RCB class 0/I versus II/III with odds ratios 7.19 (95% confidence interval [CI], 2.01-25.68; p = .002) and 8.95 (95% CI, 2.09-38.23; p = .003), respectively.
Conclusion: The combination of carboplatin and nab-paclitaxel for early stage high-risk TNBC showed manageable toxicity and encouraging antitumor activity. Immune-hot GSIS is associated with higher pCR rate and RCB class 0/1. This study provides an additional rationale for using nonanthracycline platinum-based therapy for future neoadjuvant trials in early stage TNBCs. Clinical trial identification number: NCT01525966 IMPLICATIONS FOR PRACTICE: Platinum is an important neoadjuvant chemotherapy agent for treatment of early stage triple-negative breast cancer (TNBC). In this study, carboplatin and nab-paclitaxel were well tolerated and highly effective in TNBC, resulting in pathological complete response of 48%. In univariate and multivariate analyses adjusting for age, race, tumor stage and grade, "immune-hot" GeparSixto immune signature (GSIS) and DNA repair defect (DRD) were associated with higher pathological complete response (pCR) and residual cancer burden class 0/1. The association of immune-hot GSIS with higher pCR holds promise for de-escalating neoadjuvant chemotherapy for patients with early stage TNBC. Although GSIS is not routinely used in clinic, further development of this immune signature into a clinically applicable assay is indicated.
Keywords: Carboplatin; Nab-paclitaxel; Neoadjuvant; Triple-negative breast cancer.
© 2020 AlphaMed Press.
Conflict of interest statement
Figures

References
-
- Dent R, Trudeau M, Pritchard KI et al. Triple‐negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res 2007;13:4429–4434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

