Retinal Thickness Predicts the Risk of Cognitive Decline in Parkinson Disease
- PMID: 33098308
- PMCID: PMC7756646
- DOI: 10.1002/ana.25944
Retinal Thickness Predicts the Risk of Cognitive Decline in Parkinson Disease
Abstract
Objective: This study was undertaken to analyze longitudinal changes of retinal thickness and their predictive value as biomarkers of disease progression in idiopathic Parkinson's disease (iPD).
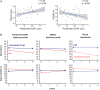
Methods: Patients with Lewy body diseases were enrolled and prospectively evaluated at 3 years, including patients with iPD (n = 42), dementia with Lewy bodies (n = 4), E46K-SNCA mutation carriers (n = 4), and controls (n = 17). All participants underwent Spectralis retinal optical coherence tomography and Montreal Cognitive Assessment, and Unified Parkinson's Disease Rating Scale score was obtained in patients. Macular ganglion cell-inner plexiform layer complex (GCIPL) and peripapillary retinal nerve fiber layer (pRNFL) thickness reduction rates were estimated with linear mixed models. Risk ratios were calculated to evaluate the association between baseline GCIPL and pRNFL thicknesses and the risk of subsequent cognitive and motor worsening, using clinically meaningful cutoffs.
Results: GCIPL thickness in the parafoveal region (1- to 3-mm ring) presented the largest reduction rate. The annualized atrophy rate was 0.63μm in iPD patients and 0.23μm in controls (p < 0.0001). iPD patients with lower parafoveal GCIPL and pRNFL thickness at baseline presented an increased risk of cognitive decline at 3 years (relative risk [RR] = 3.49, 95% confidence interval [CI] = 1.10-11.1, p = 0.03 and RR = 3.28, 95% CI = 1.03-10.45, p = 0.045, respectively). We did not identify significant associations between retinal thickness and motor deterioration.
Interpretation: Our results provide evidence of the potential use of optical coherence tomography-measured parafoveal GCIPL thickness to monitor neurodegeneration and to predict the risk of cognitive worsening over time in iPD. ANN NEUROL 2021;89:165-176.
© 2020 The Authors. Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Nothing to report.
Figures


References
-
- Zarranz JJ, Alegre J, Gomez‐Esteban JC, et al. The new mutation, E46K, of alpha‐synuclein causes Parkinson and Lewy body dementia. Ann Neurol 2004;55:164–173. - PubMed
-
- Somme JH, Gomez‐Esteban JC, Molano A, et al. Initial neuropsychological impairments in patients with the E46K mutation of the alpha‐synuclein gene (PARK 1). J Neurol Sci 2011;310:86–89. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

