Severe Acute Respiratory Distress Syndrome Secondary to Coronavirus 2 (SARS-CoV-2)
- PMID: 33098401
- PMCID: PMC7740045
- DOI: 10.34172/ijoem.2020.2202
Severe Acute Respiratory Distress Syndrome Secondary to Coronavirus 2 (SARS-CoV-2)
Abstract
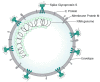
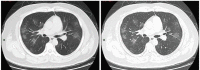
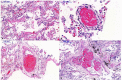
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19) and has created a worldwide pandemic. Many patients with this infection have an asymptomatic or mild illness, but a small percentage of patients require hospitalization and intensive care. Patients with respiratory tract involvement have a spectrum of presentations that range from scattered ground-glass infiltrates to diffuse infiltrates with consolidation. Patients with the latter radiographic presentation have severe hypoxemia and usually require mechanical ventilation. In addition, some patients develop multiorgan failure, deep venous thrombi with pulmonary emboli, and cytokine storm syndrome. The respiratory management of these patients should focus on using low tidal volume ventilation with low intrathoracic pressures. Some patients have significant recruitable lung and may benefit from higher positive end-expiratory pressure (PEEP) levels and/or prone positioning. There is no well-established anti-viral treatment for this infection; the United States Food and Drug Administration (FDA) has provided emergency use authorization for convalescent plasma and remdesivir for the treatment of patients with COVID-19. In addition, randomized trials have demonstrated that dexamethasone improves outcomes in patients on mechanical ventilators or on oxygen. There are ongoing trials of other drugs which have the potential to moderate the acute inflammatory state seen in some of these patients. These patients often need prolonged high-level intensive care. Hospitals are confronted with significant challenges in patient management, supply management, health care worker safety, and health care worker burnout.
Keywords: 2019-nCoV; COVID 19; Coronavirus; Hypoxia; Middle East respiratory syndrome coronavirus; Novel coronavirus; Remdesivir; SARS coronavirus.
Conflict of interest statement
None declared.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous