COVID-19 associated coagulopathy in critically ill patients: A hypercoagulable state demonstrated by parameters of haemostasis and clot waveform analysis
- PMID: 33098540
- PMCID: PMC7584863
- DOI: 10.1007/s11239-020-02318-x
COVID-19 associated coagulopathy in critically ill patients: A hypercoagulable state demonstrated by parameters of haemostasis and clot waveform analysis
Abstract
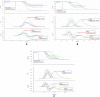
Patients with COVID-19 are known to be at risk of developing both venous, arterial and microvascular thrombosis, due to an excessive immuno-thrombogenic response to the SARS-CoV-2 infection. Overlapping syndromes of COVID-19 associated coagulopathy with consumptive coagulopathy and microangiopathy can be seen in critically ill patients as well. Blood was collected from 12 Intensive Care Unit (ICU) patients with severe COVID-19 who were on either mechanical ventilation or on high flow oxygen with a PaO2/FiO2 ratio of <300 mmHg. Laboratory tests were performed for parameters of haemostasis, clot waveform analysis and anti-phospholipid antibodies. CWA parameters were raised with elevated aPTT median Min1 (clot velocity) 9.3%/s (IQR 7.1-9.9%/s), elevated PT median Min1 10.3%/s (IQR 7.1-11.1%/s), elevated aPTT median Min2 (clot acceleration) 1.5%/s2 (IQR 1.0-1.6%/s2), elevated PT median Min2 5.2%/s2 (3.6-5.7%/s2), elevated aPTT median Max2 (clot deceleration) 1.3%/s2 (IQR 0.8-1.4%/s2) elevated PT median Max2 3.8%/s2 (IQR 2.6-4.2%/s2), increased aPTT median Delta change (decreased light transmission due to increased clot formation) 87.8% (IQR 70.2-91.8%) and PT median Delta change 33.0%. This together with raised median Factor VIII levels of 262.5%, hyperfibrinogenemia (median fibrinogen levels 7.5 g/L), increased median von Willebrand factor antigen levels 320% and elevated median D-dimer levels 1.7 μg/dl support the diagnosis of COVID-19 associated coagulopathy. A lupus anticoagulant was present in 50% of patients. Our laboratory findings further support the view that severe SARS-CoV-2 infection is associated with a state of hypercoagulability.
Keywords: Coronavirus; Hypercoagulability; Sepsis; Thrombophilia; Thrombosis.
Conflict of interest statement
The authors declare that they have no conflict of interest. No funding for this study was obtained.
Figures
References
-
- Hunt B, Retter A, McClintock C (2020) Practical guidance for the prevention of thrombosis and management of coagulopathy and disseminated intravascular coagulation of patients infected with COVID-19. https://b-s-h.org.uk/media/18171/th-and-covid-25-march-2020-final.pdf
-
- Kreuziger LB, Lee A, Garcia D, et al (2020) COVID-19 and VTE/anticoagulation: frequently asked questions (Version 4.0; last reviewed July 20, 2020) https://www.hematology.org/covid-19/covid-19-and-vte-anticoagulation
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

