Effects of intensive versus standard blood pressure control on domain-specific cognitive function: a substudy of the SPRINT randomised controlled trial
- PMID: 33098800
- PMCID: PMC7714000
- DOI: 10.1016/S1474-4422(20)30319-7
Effects of intensive versus standard blood pressure control on domain-specific cognitive function: a substudy of the SPRINT randomised controlled trial
Abstract
Background: Results from the Systolic Blood Pressure Intervention Trial (SPRINT) showed that intensive control of systolic blood pressure significantly reduced the occurrence of mild cognitive impairment, but not probable dementia. We investigated the effects of intensive lowering of systolic blood pressure on specific cognitive functions in a preplanned substudy of participants from SPRINT.
Methods: SPRINT was an open-label, multicentre, randomised controlled trial undertaken at 102 sites, including academic medical centres, Veterans Affairs medical centres, hospitals, and independent clinics, in the USA and Puerto Rico. Participants were adults aged 50 years or older with systolic blood pressure higher than 130 mm Hg, but without diabetes, history of stroke, or dementia. Participants were randomly assigned (1:1) to a systolic blood pressure goal of less than 120 mm Hg (intensive treatment) versus less than 140 mm Hg (standard treatment). All major classes of antihypertensive agents were included. A subgroup of randomly assigned participants including, but not limited to, participants enrolled in an MRI substudy was then selected for a concurrent substudy of cognitive function (target 2800 participants). Each individual was assessed with a screening cognitive test battery and an extended cognitive test battery at baseline and biennially during the planned 4-year follow-up. The primary outcomes for this substudy were standardised composite scores for memory (Logical Memory I and II, Modified Rey-Osterrieth Complex Figure [immediate recall], and Hopkins Verbal Learning Test-Revised [delayed recall]) and processing speed (Trail Making Test and Digit Symbol Coding). SPRINT was registered with ClinicalTrials.gov, NCT01206062.
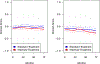
Findings: From Nov 23, 2010, to Dec 28, 2012, 2921 participants (mean age 68·4 years [SD 8·6], 1080 [37%] women) who had been randomly assigned in SPRINT were enrolled in the substudy (1448 received intensive treatment and 1473 received standard treatment). SPRINT was terminated early due to benefit observed in the primary outcome (composite of cardiovascular events). After a median follow-up of 4·1 years (IQR 3·7-5·8), there was no between-group difference in memory, with an annual decline in mean standardised domain score of -0·005 (95% CI -0·010 to 0·001) in the intensive treatment group and -0·001 (-0·006 to 0·005) in the standard treatment group (between-group difference -0·004, 95% CI -0·012 to 0·004; p=0·33). Mean standardised processing speed domain scores declined more in the intensive treatment group (between-group difference -0·010, 95% CI -0·017 to -0·002; p=0·02), with an annual decline of -0·025 (-0·030 to -0·019) for the intensive treatment group and -0·015 (-0·021 to 0·009) for the standard treatment group.
Interpretation: Intensive treatment to lower systolic blood pressure did not result in a clinically relevant difference compared with standard treatment in memory or processing speed in a subgroup of participants from SPRINT. The effect of blood pressure lowering might not be evident in specific domains of cognitive function, but instead distributed across multiple domains.
Funding: National Heart, Lung, and Blood Institute, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute on Aging, National Institute of Neurological Disorders and Stroke, and the Alzheimer's Association.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Cognitive function in SPRINT: where do we go next?Lancet Neurol. 2020 Nov;19(11):880-881. doi: 10.1016/S1474-4422(20)30356-2. Lancet Neurol. 2020. PMID: 33098785 No abstract available.
References
-
- Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003; 348(13): 1215–22. - PubMed
-
- Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol 2014; 13(8): 788–94. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UL1 TR000433/TR/NCATS NIH HHS/United States
- UL1 TR000064/TR/NCATS NIH HHS/United States
- UL1 TR000075/TR/NCATS NIH HHS/United States
- UL1 TR002538/TR/NCATS NIH HHS/United States
- UL1 TR000003/TR/NCATS NIH HHS/United States
- UL1 TR000050/TR/NCATS NIH HHS/United States
- UL1 RR025755/RR/NCRR NIH HHS/United States
- R01 AG065805/AG/NIA NIH HHS/United States
- R01 AG055606/AG/NIA NIH HHS/United States
- P30 AG021332/AG/NIA NIH HHS/United States
- K01 HL133468/HL/NHLBI NIH HHS/United States
- UL1 TR000445/TR/NCATS NIH HHS/United States
- HHSN268200900048C/HL/NHLBI NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- HHSN268200900040C/HL/NHLBI NIH HHS/United States
- UL1 TR002548/TR/NCATS NIH HHS/United States
- HHSN268200900046C/HL/NHLBI NIH HHS/United States
- P30 GM103337/GM/NIGMS NIH HHS/United States
- UL1 TR001064/TR/NCATS NIH HHS/United States
- UL1 RR025752/RR/NCRR NIH HHS/United States
- UL1 RR025771/RR/NCRR NIH HHS/United States
- K23 NS107645/NS/NINDS NIH HHS/United States
- UL1 TR000093/TR/NCATS NIH HHS/United States
- U54 GM104940/GM/NIGMS NIH HHS/United States
- P30 AG049638/AG/NIA NIH HHS/United States
- HHSN268200900049C/HL/NHLBI NIH HHS/United States
- HHSN268200900047C/HL/NHLBI NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- UL1 TR000073/TR/NCATS NIH HHS/United States
- UL1 TR000002/TR/NCATS NIH HHS/United States
- UL1 TR000105/TR/NCATS NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- UL1 TR003142/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

