PET imaging of neuroinflammation in neurological disorders
- PMID: 33098803
- PMCID: PMC7912433
- DOI: 10.1016/S1474-4422(20)30346-X
PET imaging of neuroinflammation in neurological disorders
Abstract
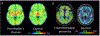
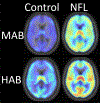
A growing need exists for reliable in-vivo measurement of neuroinflammation to better characterise the inflammatory processes underlying various diseases and to inform the development of novel therapeutics that target deleterious glial activity. PET is well suited to quantify neuroinflammation and has the potential to discriminate components of the neuroimmune response. However, there are several obstacles to the reliable quantification of neuroinflammation by PET imaging. Despite these challenges, PET studies have consistently identified associations between neuroimmune responses and pathophysiology in brain disorders such as Alzheimer's disease. Tissue studies have also begun to clarify the meaning of changes in PET signal in some diseases. Furthermore, although PET imaging of neuroinflammation does not have an established clinical application, novel targets are under investigation and a small but growing number of studies have suggested that this imaging modality could have a role in drug development. Future studies are needed to further improve our knowledge of the cellular mechanisms that underlie changes in PET signal, how immune response contributes to neurological disease, and how it might be therapeutically modified.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures


References
-
- Colonna M, Wang Y. TREM2 variants: new keys to decipher Alzheimer disease pathogenesis. Nat Rev Neurosci 2016; 17: 201–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

