Seroconversion in patients with cancer and oncology health care workers infected by SARS-CoV-2
- PMID: 33098994
- PMCID: PMC7577226
- DOI: 10.1016/j.annonc.2020.10.473
Seroconversion in patients with cancer and oncology health care workers infected by SARS-CoV-2
Abstract
Background: Patients with cancer have high risk for severe complications and poor outcome to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-related disease [coronavirus disease 2019 (COVID-19)]. Almost all subjects with COVID-19 develop anti-SARS-CoV-2 immunoglobulin G (IgG) within 3 weeks after infection. No data are available on the seroconversion rates of cancer patients and COVID-19.
Patients and methods: We conducted a multicenter, observational, prospective study that enrolled (i) patients and oncology health professionals with SARS-CoV-2 infection confirmed by real-time RT-PCR assays on nasal/pharyngeal swab specimens; (ii) patients and oncology health professionals with clinical or radiological suspicious of infection by SARS-CoV-2; and (iii) patients with cancer who are considered at high risk for infection and eligible for active therapy and/or major surgery. All enrolled subjects were tested with the 2019-nCoV IgG/IgM Rapid Test Cassette, which is a qualitative membrane-based immunoassay for the detection of IgG and IgM antibodies to SARS-CoV-2. The aim of the study was to evaluate anti-SARS-CoV-2 seroconversion rate in patients with cancer and oncology health care professionals with confirmed or clinically suspected COVID-19.
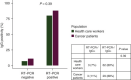
Results: From 30 March 2020 to 11 May 2020, 166 subjects were enrolled in the study. Among them, cancer patients and health workers were 61 (36.7%) and 105 (63.3%), respectively. Overall, 86 subjects (51.8%) had confirmed SARS-CoV-2 diagnosis by RT-PCR testing on nasopharyngeal swab specimen, and 60 (36.2%) had a clinical suspicious of COVID-19. Median time from symptom onset (for cases not confirmed by RT-PCR) or RT-PCR confirmation to serum antibody test was 17 days (interquartile range 26). In the population with confirmed RT-PCR, 83.8% of cases were IgG positive. No difference in IgG positivity was observed between cancer patients and health workers (87.9% versus 80.5%; P = 0.39).
Conclusions: Our data indicate that SARS-CoV-2-specific IgG antibody detection do not differ between cancer patients and healthy subjects.
Keywords: COVID-19; SARS-CoV-2; antibody response; cancer; coronavirus; seroconversion.
Copyright © 2020 European Society for Medical Oncology. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosure DG reports personal fees for consulting, advisory role and speakers' bureau from Novartis, Pfizer, Lilly; fees for travel and accommodations from Novartis, Pfizer, Lilly. GC reports personal fees for consulting, advisory role, and speakers' bureau from Roche/Genentech, Novartis, Pfizer, Lilly, Foundation Medicine, Samsung, and Daichii-Sankyo; honoraria from Ellipses Pharma; fees for travel and accommodations from Roche/Genentech, and Pfizer. The other authors have declared no conflicts of interest. Data sharing All data generated or analyzed during this study are included in the published article. Additional supporting data are available from the corresponding author on reasonable request. All requests for raw and analyzed data and materials will be reviewed by the corresponding author to verify whether the request is subject to any intellectual property or confidentiality obligations.
Figures



References
-
- World Health Organization Coronavirus disease 2019 (COVID-19) Situation Report - 191. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2... Available at:
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

