On the mechanisms underlying attenuated redox responses to exercise in older individuals: A hypothesis
- PMID: 33099002
- PMCID: PMC7754707
- DOI: 10.1016/j.freeradbiomed.2020.10.026
On the mechanisms underlying attenuated redox responses to exercise in older individuals: A hypothesis
Abstract
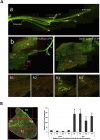
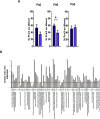
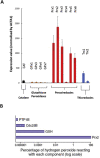
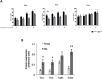
Responding appropriately to exercise is essential to maintenance of skeletal muscle mass and function at all ages and particularly during aging. Here, a hypothesis is presented that a key component of the inability of skeletal muscle to respond effectively to exercise in aging is a denervation-induced failure of muscle redox signalling. This novel hypothesis proposes that an initial increase in oxidation in muscle mitochondria leads to a paradoxical increase in the reductive state of specific cysteines of signalling proteins in the muscle cytosol that suppresses their ability to respond to normal oxidising redox signals during exercise. The following are presented for consideration:Transient loss of integrity of peripheral motor neurons occurs repeatedly throughout life and is normally rapidly repaired by reinnervation, but this repair process becomes less efficient with aging. Each transient loss of neuromuscular integrity leads to a rapid, large increase in mitochondrial peroxide production in the denervated muscle fibers and in neighbouring muscle fibers. This peroxide may initially act to stimulate axonal sprouting and regeneration, but also stimulates retrograde mitonuclear communication to increase expression of a range of cytoprotective proteins in an attempt to protect the fiber and neighbouring tissues against oxidative damage. The increased peroxide within mitochondria does not lead to an increased cytosolic peroxide, but the increases in adaptive cytoprotective proteins include some located to the muscle cytosol which modify the local cytosol redox environment to induce a more reductive state in key cysteines of specific signalling proteins. Key adaptations of skeletal muscle to exercise involve transient peroxiredoxin oxidation as effectors of redox signalling in the cytosol. This requires sensitive oxidation of key cysteine residues. In aging, the chronic change to a more reductive cytosolic environment prevents the transient oxidation of peroxiredoxin 2 and hence prevents essential adaptations to exercise, thus contributing to loss of muscle mass and function. Experimental approaches suitable for testing the hypothesis are also outlined.
Keywords: Aging; Exercise; Muscle; Redox; Training.
Copyright © 2020. Published by Elsevier Inc.
Figures




Similar articles
-
Redox responses in skeletal muscle following denervation.Redox Biol. 2019 Sep;26:101294. doi: 10.1016/j.redox.2019.101294. Epub 2019 Aug 8. Redox Biol. 2019. PMID: 31450104 Free PMC article.
-
Exercise stress leads to an acute loss of mitochondrial proteins and disruption of redox control in skeletal muscle of older subjects: An underlying decrease in resilience with aging?Free Radic Biol Med. 2021 Dec;177:88-99. doi: 10.1016/j.freeradbiomed.2021.10.003. Epub 2021 Oct 14. Free Radic Biol Med. 2021. PMID: 34655746
-
2-Cys peroxiredoxin oxidation in response to hydrogen peroxide and contractile activity in skeletal muscle: A novel insight into exercise-induced redox signalling?Free Radic Biol Med. 2020 Nov 20;160:199-207. doi: 10.1016/j.freeradbiomed.2020.06.020. Epub 2020 Aug 9. Free Radic Biol Med. 2020. PMID: 32784030 Free PMC article.
-
Redox regulation of muscle adaptations to contractile activity and aging.J Appl Physiol (1985). 2015 Aug 1;119(3):163-71. doi: 10.1152/japplphysiol.00760.2014. Epub 2015 Mar 19. J Appl Physiol (1985). 2015. PMID: 25792715 Free PMC article. Review.
-
Muscle redox signalling pathways in exercise. Role of antioxidants.Free Radic Biol Med. 2016 Sep;98:29-45. doi: 10.1016/j.freeradbiomed.2016.02.022. Epub 2016 Feb 18. Free Radic Biol Med. 2016. PMID: 26912034 Review.
Cited by
-
Hallmarks of ageing in human skeletal muscle and implications for understanding the pathophysiology of sarcopenia in women and men.Clin Sci (Lond). 2023 Nov 29;137(22):1721-1751. doi: 10.1042/CS20230319. Clin Sci (Lond). 2023. PMID: 37986616 Free PMC article.
-
Effects of 3-(4-Hydroxy-3-methoxyphenyl)propionic Acid on Regulating Oxidative Stress and Muscle Fiber Composition.Nutrients. 2025 Feb 13;17(4):668. doi: 10.3390/nu17040668. Nutrients. 2025. PMID: 40004996 Free PMC article.
-
Reactive oxygen species in the pathogenesis of sarcopenia.Free Radic Biol Med. 2025 Feb 1;227:446-458. doi: 10.1016/j.freeradbiomed.2024.11.046. Epub 2024 Nov 28. Free Radic Biol Med. 2025. PMID: 39613046 Free PMC article. Review.
-
Redox Control of Skeletal Muscle Function and Adaptations to Exercise.Adv Exp Med Biol. 2025;1478:459-473. doi: 10.1007/978-3-031-88361-3_19. Adv Exp Med Biol. 2025. PMID: 40879951 Review.
-
Mitochondria as Nutritional Targets to Maintain Muscle Health and Physical Function During Ageing.Sports Med. 2024 Sep;54(9):2291-2309. doi: 10.1007/s40279-024-02072-7. Epub 2024 Jul 26. Sports Med. 2024. PMID: 39060742 Free PMC article. Review.
References
-
- Leveille S.G. Musculoskeletal aging. Curr. Opin. Rheumatol. 2004;16(2):114–118. - PubMed
-
- Laurin D., Verreault R., Lindsay J., MacPherson K., Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch. Neurol. 2001;58(3):498–504. - PubMed
-
- Young A., Skelton D.A. Applied physiology of strength and power in old age. Int. J. Sports Med. 1994;15(3):149–151. - PubMed
-
- Porter M.M., Vandervoort A.A., Lexell J. Aging of human muscle: structure, function and adaptability. Scand. J. Med. Sci. Sports. 1995;5(3):129–142. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

