Ex vivo radiosensitivity is increased in non-cancer patients taking valproate
- PMID: 33099323
- PMCID: PMC7585294
- DOI: 10.1186/s12883-020-01966-z
Ex vivo radiosensitivity is increased in non-cancer patients taking valproate
Abstract
Background: Valproate (VPA) is a commonly prescribed antiepileptic drug for patients experiencing epileptic seizures due to brain tumors. VPA increases radiation sensitivity in various tumor cells in vitro due to complex mechanisms. This could make tumors more vulnerable to ionizing radiation or overcome radioresistance. Yet, clinical data on possible improvement of tumor control by adding VPA to tumor therapy is controversial. Potentially radiosensitizing effects of VPA on healthy tissue remain unclear. To determine individual radiosensitivity, we analyzed blood samples of individuals taking VPA.
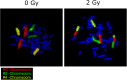
Methods: Ex vivo irradiated blood samples of 31 adult individuals with epilepsy were studied using 3-color fluorescence in situ hybridization. Aberrations in chromosomes 1, 2 and 4 were analyzed. Radiosensitivity was determined by the mean breaks per metaphase (B/M) and compared to age-matched (2:1) healthy donors.
Results: The patient cohort (n = 31; female: 38.7%) showed an increase of their average B/M value compared to healthy individuals (n = 61; female: 56.9%; B/M: 0.480 ± 0.09 vs. 0.415 ± 0.07; p = .001). The portion of radiosensitive (B/M > 0.500) and distinctly radiosensitive individuals (B/M > 0.600) was increased in the VPA group (54.9% vs. 11.3 and 9.7% vs. 0.0%; p < .001). In 3/31 patients, radiosensitivity was determined prior to and after VPA treatment and radiosensitivity was increased by VPA-treatment.
Conclusions: In our study, we confirmed that patients treated with VPA had an increased radiosensitivity compared to the control group. This could be considered in patients taking VPA prior to the beginning of radiotherapy to avoid toxic side effects of VPA-treatment.
Keywords: 3-colour fluorescence-in-situ-hybridization; Breaks per metaphase; Individual radiosensitivity; Valproate.
Conflict of interest statement
WG received a lecture fee from UCB and Desitin. HH reports personal fees from UCB, personal fees from Desitin, personal fees from Eisai, personal fees from GW, personal fees from Novartis, personal fees from IQWiG, personal fees from Hexal, personal fees from facetoface, grants from Amgen, Ad-Tech, Bracco, Pfizer, Micromed, Nihon Kohden, personal fees from Arvelle, outside the submitted work. The other authors have nothing to report.
Figures

References
-
- Duenas-Gonzalez A, Candelaria M, Perez-Plascencia C, Perez-Cardenas E, de la Cruz-Hernandez E, Herrera LA. Valproic acid as epigenetic cancer drug: preclinical, clinical and transcriptional effects on solid tumors. Cancer Treat Rev 2008;34(3):206–222. - PubMed
-
- Gottlicher M. Valproic acid: an old drug newly discovered as inhibitor of histone deacetylases. Ann Hematol. 2004;83(Suppl 1):S91–S92. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

