Plasma deoxyuridine as a surrogate marker for toxicity and early clinical response in patients with metastatic colorectal cancer after 5-FU-based therapy in combination with arfolitixorin
- PMID: 33099678
- PMCID: PMC7801297
- DOI: 10.1007/s00280-020-04173-2
Plasma deoxyuridine as a surrogate marker for toxicity and early clinical response in patients with metastatic colorectal cancer after 5-FU-based therapy in combination with arfolitixorin
Abstract
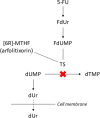
Purpose: The aim was to explore the correlation between increasing doses of [6R]-5,10-methylenetetrahydrofolate (arfolitixorin) and plasma concentrations of deoxyuridine (dUr) in patients with metastatic colorectal cancer (mCRC), subjected to 5-fluorouracil (5-FU)-based chemotherapy. The aim was further to investigate the possibility to predict toxicity and clinical response during treatment using gender, age, and plasma dUr as explanatory variables.
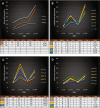
Methods: Thirty-three patients from the ISO-CC-005 phase I/IIa study, which investigated safety and tolerability of arfolitixorin at four dose levels, were included. Toxicity and clinical response were evaluated after 4 cycles of chemotherapy. Plasma dUr was quantified before (0 h) and 24 h after 5-FU administration at the first (C1) and fourth (C4) cycle using LC-MS/MS. Fit modelling was used to predict toxicity and clinical response.
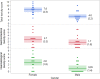
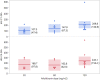
Results: The dUr levels increased with increasing arfolitixorin dose. Females had higher total and haematological toxicity scores (p = 0.0004 and 0.0089, respectively), and needed dose reduction more often than males (p = 0.012). Fit modeling showed that gender and the dUr levels at C1-0 h and C4-24 h predicted total toxicity (p = 0.0011), whereas dUr C4-0 h alone was associated with gastrointestinal toxicity (p = 0.026). Haematological toxicity was predicted by gender and age (p = 0.0071). The haematological toxicity score in combination with the dUr levels at C1-24 h and C4-24 h predicted early clinical response (p = 0.018).
Conclusion: The dUr level before and during administration of 5-FU and arfolitixorin was predictive for toxicity and early clinical response and could be a potential surrogate marker for thymidylate synthase inhibition in patients with mCRC.
Trial registration: NCT02244632, first posted on ClinicalTrials.gov on September 19, 2014.
Keywords: 5-Fluorouracil; Folate; Gastrointestinal toxicity; Gender; Haematological toxicity; Metastatic.
Conflict of interest statement
RT is an employee of Isofol. BG and EO are stockholders of Isofol. BG and HT are immediate family members. Other authors declare no competing interests.
Figures
References
-
- van de Velde CJ, Boelens PG, Borras JM, Coebergh JW, Cervantes A, Blomqvist L, Beets-Tan RG, van den Broek CB, Brown G, Van Cutsem E, Espin E, Haustermans K, Glimelius B, Iversen LH, van Krieken JH, Marijnen CA, Henning G, Gore-Booth J, Meldolesi E, Mroczkowski P, Nagtegaal I, Naredi P, Ortiz H, Pahlman L, Quirke P, Rodel C, Roth A, Rutten H, Schmoll HJ, Smith JJ, Tanis PJ, Taylor C, Wibe A, Wiggers T, Gambacorta MA, Aristei C, Valentini V (2014) EURECCA colorectal: multidisciplinary management: European consensus conference colon and rectum. Eur J Cancer 50 (1):1 e1–1 e34. 10.1016/j.ejca.2013.06.048. - PubMed
-
- Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Kohne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Osterlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taieb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422. doi: 10.1093/annonc/mdw235. - DOI - PubMed
-
- Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, Nagtegaal ID, Beets-Tan RG, Arnold D, Ciardiello F, Hoff P, Kerr D, Kohne CH, Labianca R, Price T, Scheithauer W, Sobrero A, Tabernero J, Aderka D, Barroso S, Bodoky G, Douillard JY, El Ghazaly H, Gallardo J, Garin A, Glynne-Jones R, Jordan K, Meshcheryakov A, Papamichail D, Pfeiffer P, Souglakos I, Turhal S, Cervantes A (2012) ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol 23 (10):2479–2516. 10.1093/annonc/mds236. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

