Immunosuppressive regimens for adult liver transplant recipients in real-life practice: consensus recommendations from an Italian Working Group
- PMID: 33099753
- PMCID: PMC7803715
- DOI: 10.1007/s12072-020-10091-5
Immunosuppressive regimens for adult liver transplant recipients in real-life practice: consensus recommendations from an Italian Working Group
Erratum in
-
Correction to: Immunosuppressive regimens for adult liver transplant recipients in real-life practice: consensus recommendations from an Italian Working Group.Hepatol Int. 2021 Jun;15(3):831. doi: 10.1007/s12072-021-10178-7. Hepatol Int. 2021. PMID: 33779872 Free PMC article. No abstract available.
Abstract
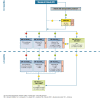
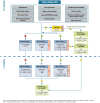
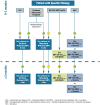
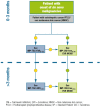
It is a well-recognized fact that implementing new guidelines in clinical practice may be difficult; therefore the Italian Society for Organ and Tissue Transplantation (SITO) set out to define practical immunosuppression tools for the management of liver transplantation patients. In 2017, an Italian Working Group of liver transplant experts and hepatologists issued a set of consensus statements along with evidence-based recommendations on the use of everolimus after liver transplantation. This article presents the evidence- and consensus-based algorithms developed within the Italian Working Group, which are aimed towards guiding clinicians in the selection of immunosuppressive regimens for the management of adult liver transplant recipients in real-life practice. The liver transplant recipient population, typically managed in clinical practice, was divided into the following categories: (1) standard patients; (2) critically ill patients; (3) patients with a specific etiology; (4) patients with hepatocellular carcinoma; (5) and patients with de novo malignancies. The algorithms are divided into two parts, according to the time from transplantation (0-3 months and > 3 months) and are discussed here along with relevant supporting literature, when available. Ultimately, it is hoped that the evidence- and consensus-based algorithms developed within the Italian Working Group, and presented here, contribute to simplify, personalize, and optimize immunosuppression of liver transplantation recipients in clinical practice.
Keywords: Calcineurin inhibitor; Chronic kidney disease; Consensus recommendations; Everolimus; Hepatocellular carcinoma; Immunosuppression; Sirolimus; Solid-organ transplantation; Tacrolimus; mTOR inhibitor.
Conflict of interest statement
Umberto Cillo declares that he has no conflict of interest. Luciano De Carlis declares that he has no conflict of interest. Massimo Del Gaudio declares that he has no conflict of interest. Paolo De Simone served as an advisory board member for Novartis, Astellas and Chiesi. Stefano Fagiuoli Advisory Board and Speaker’s Bureau for Abbvie, Gilead Sciences, MSD, Novartis, Astellas, Bayer, Kedrion, Intercept. Francesco Lupo served as advisory board member for Novartis, Astellas, Biotest, Chiesi. Giuseppe Tisone declares that he has no conflict of interest. Riccardo Volpes declares that he has no conflict of interest.
Figures

References
-
- Nashan B. mTOR Inhibition and clinical transplantation: liver. Transplantation. 2018;102:S19–S26. - PubMed
-
- Gonwa TA, Mai ML, Melton LB, Hays SR, Goldstein RM, Levy MF, et al. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: risk of development and treatment. Transplantation. 2001;72:1934–1939. - PubMed
-
- Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397:530–534. - PubMed
-
- Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

