What are the patients' and health care professionals' understanding and behaviors towards adverse drug reaction reporting and additional monitoring?
- PMID: 33099846
- PMCID: PMC7894330
- DOI: 10.1002/pds.5162
What are the patients' and health care professionals' understanding and behaviors towards adverse drug reaction reporting and additional monitoring?
Abstract
Background: The additional monitoring (AM)/black triangle concept is aimed to enhance ADR reporting for certain types of medicinal products for which the safety profile is less well established.
Purpose: The objective of this survey was to assess (a) attitudes towards ADR reporting and reasons for not reporting an ADR and (b) awareness of AM among HCPs, patients or their careers in EU countries.
Methods: An online questionnaire which was available in all EU languages was completed by 2918 responders coming from all EEA countries.
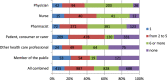
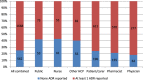
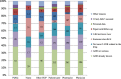
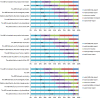
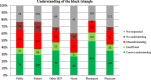
Results: The main factors motivating to report an ADR were severity or novelty of the reaction or novelty of the medicine. The main factors for not reporting an ADR was the fact that the ADR is already known (35%), the ADR was not serious (18%) or reporter was not sure if the ADR was related to the medicine (15%). Half of the respondents indicated that they have seen AM statement before. Thirty percent of the responders had correct understanding of the AM concept while 20 % misunderstood the concept.
Conclusion: Underreporting occurs but it seems this is because of reporter's prioritisation towards certain type of ADRs. AM aims to increase reporting for certain medicines, however, approximately half of responders have seen the AM symbol before and 20% of all responders (independent of their previous awareness) misunderstood the concept.
Keywords: additional monitoring; adverse drug reactions; black triangle; impact; pharmacoepidemiology; pharmacovigilance.
© 2020 The Authors. Pharmacoepidemiology and Drug Safety published by John Wiley & Sons Ltd.
Conflict of interest statement
The views expressed in this article are the personal views of the author(s) and may not be understood or quoted as being made on behalf of or reflecting the position of the EMA or one of its committees or working parties.
Figures


References
-
- Hazell L, Shakir SAW. Under‐reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385‐396. - PubMed
-
- Guideline on good pharmacovigilance practices Module X—Additional monitoring. Retrieved from. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin...
-
- Regulation (EC) No 726/2004 of the European Parliament and of the council of 31 March laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency, Official Journal L–136, p. 1–33. Retrieved from http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:136:0001...
-
- Report from the Commission to the European Parliament and the Council on the national and European Medicines Agency experience regarding the list of medicines for human use subject to additional monitoring. Retrieved from https://ec.europa.eu/transparency/regdoc/rep/1/2019/EN/COM-2019-591-F1-E...
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

