Thioallenoates in Catalytic Enantioselective [2+2]-Cycloadditions with Unactivated Alkenes
- PMID: 33100416
- PMCID: PMC7584306
- DOI: 10.1016/j.tet.2019.04.028
Thioallenoates in Catalytic Enantioselective [2+2]-Cycloadditions with Unactivated Alkenes
Abstract
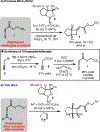
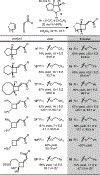
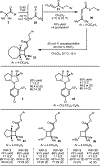
The application of thioallenoates to catalytic enantioselective [2+2]-cycloadditions with unactivated alkenes is reported.In many cases, the thioallenoates examined exhibit superior reactivity and selectivity compared to the alkoxy analogs generally used in these cycloadditions.
Keywords: Allenes; Cycloaddition; Cyclobutane; Enantioselective; Lewis-Acid.
Figures

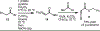
References
-
- Brimioulle R; Lenhart D; Maturi MM; Bach T Enantioselective Catalysis of Photochemical Reactions. Angew. Chem. Int. Ed. Engl 2015, 54 (13), 3872–3890. - PubMed
- Blum TR; Miller ZD; Bates DM; Guzei IA; Yoon TP Enantioselective Photochemistry Through Lewis Acid-Catalyzed Triplet Energy Transfer. Science 2016, 354 (6318), 1391–1395. - PMC - PubMed
- Poplata S; Bach T Enantioselective Intermolecular [2+2] Photocycloaddition Reaction of Cyclic Enones and Its Application in a Synthesis of (−)-Grandisol. J. Am. Chem. Soc 2018, 140 (9), 3228–3231. - PMC - PubMed
-
- Ishihara K; Nakano K Enantioselective [2 + 2] Cycloaddition of Unactivated Alkenes with Α-Acyloxyacroleins Catalyzed by Chiral Organoammonium Salts. J. Am. Chem. Soc 2007, 129 (29), 8930–8931. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
