Synthesis of an Oxathiolane Drug Substance Intermediate Guided by Constraint-Driven Innovation
- PMID: 33100812
- PMCID: PMC7574620
- DOI: 10.1021/acs.oprd.0c00145
Synthesis of an Oxathiolane Drug Substance Intermediate Guided by Constraint-Driven Innovation
Abstract
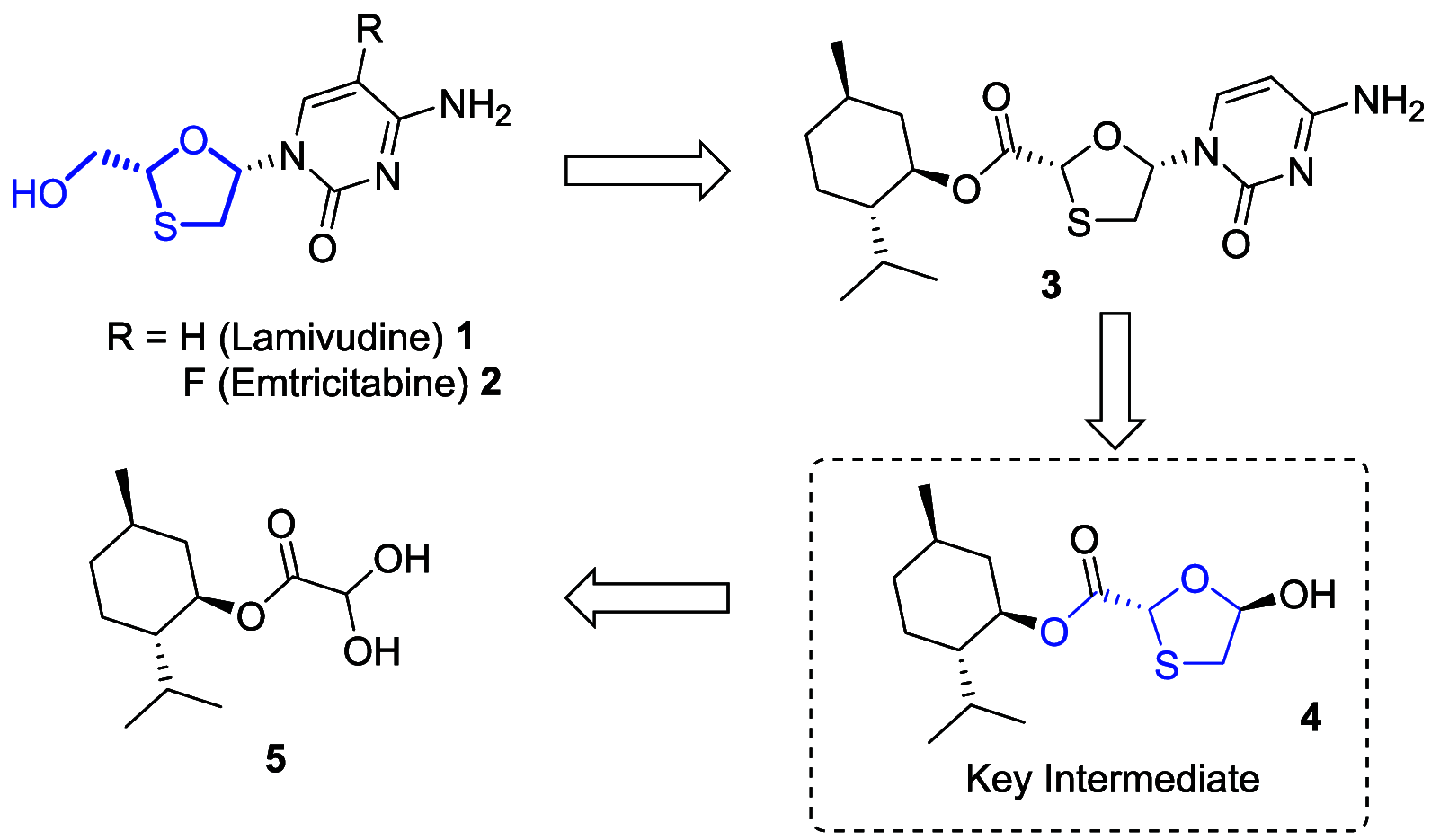
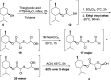
A new route was developed for construction of the oxathiolane intermediate used in the synthesis of lamivudine (3TC) and emtricitabine (FTC). We developed the presented route by constraining ourselves to low-cost, widely available starting materials-we refer to this as supply-centered synthesis. Sulfenyl chloride chemistry was used to construct the framework for the oxathiolane from acyclic precursors. This bond construction choice enabled the use of chloroacetic acid, vinyl acetate, sodium thiosulfate, and water to produce the oxathiolane.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


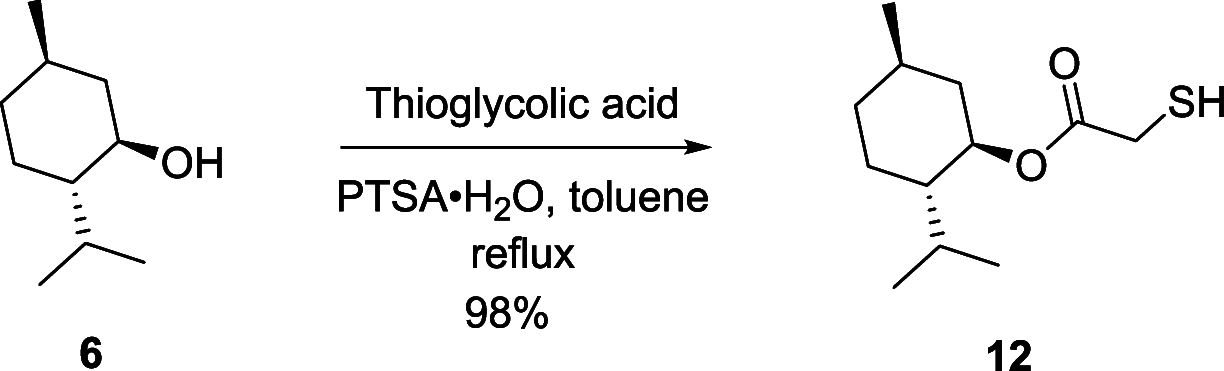
References
-
- Panos Z.; Fox J.; Prabhu V.. HIV Market Report: The State of HIV Treatment, Testing, and Prevention in Low- and Middle-Income Countries, issue (10), . Clinton Health Access Initiative, September 2019. http://clintonhealthaccess.org/wp-content/uploads/2019/12/2019-HIV-Marke... (accessed 2020-02-06).
-
- Humber D. C.; Jones M. F.; Payne J. J.; Ramsay M. V. J.; Zacharie B.; Jin H.; Siddiqui A.; Evans C. A.; Tse H. L. A.; Mansour T. S. Expeditious preparation of (−)-2′-deoxy-3′-thiacytidine (3TC). Tetrahedron Lett. 1992, 33, 4625–4628. 10.1016/S0040-4039(00)61330-8. - DOI
- Hoong L. K.; Strange L. E.; Liotta D. C.; Koszalka G. W.; Burns C. L.; Schinazi R. F. Enzyme-mediated enantioselective preparation of pure enantiomers of the antiviral agent 2′,3′-dideoxy-5-fluoro-3′-thiacytidine (FTC) and related compounds. J. Org. Chem. 1992, 57, 5563–5565. 10.1021/jo00047a004. - DOI
- Jeong L. S.; Schinazi R. F.; Beach W. J.; Kim H. O.; Nampalli S.; Shanmuganathan K.; Alves A. J.; McMillan A.; Chu C. K.; Mathis R. Asymmetric synthesis and biological evaluation of β-l-(2R,5S)- and α-l-(2R,5R)-1,3-oxathiolane-pyrimidine and -purine nucleosides as potential anti-HIV agents. J. Med. Chem. 1993, 36, 181–195. 10.1021/jm00054a001. - DOI - PubMed
- Mahmoudian M.; Baines B. S.; Drake C. S.; Hale R. S.; Jones P.; Piercey J. E.; Montgomery D. S.; Purvis I. J.; Storer R.; Dawson M. J.; Lawrence G. C. Enzymatic production of optically pure (2′R-cis)-2′-deoxy-3′-thiacytidine (3TC, lamivudine): A potent anti-HIV agent. Enzyme Microb. Technol. 1993, 15, 749–755. 10.1016/0141-0229(93)90005-M. - DOI - PubMed
- Milton J.; Brand S.; Jones M. F.; Rayner C. M. Enzymatic Resolution of α-acetoxysulfides: A new approach to the synthesis of homochiral S, O – Acetals. Tetrahedron: Asymmetry 1995, 6, 1903–1906. 10.1016/0957-4166(95)00248-N. - DOI
- Milton J.; Brand S.; Jones M. F.; Rayner C. M. Enantioselective enzymatic synthesis of the anti-viral agent lamivudine (3TC). Tetrahedron Lett. 1995, 36, 6961–6964. 10.1016/0040-4039(95)01380-Z. - DOI
- Cousins R. P. C.; Mahmoudian M.; Youds P. M. Enzymic resolution of oxathiolane intermediates - an alternative approach to the anti-viral agent lamivudine (3TC). Tetrahedron: Asymmetry 1995, 6, 393–396. 10.1016/0957-4166(95)00022-H. - DOI
- Jin H.; Siddiqui A.; Evans C. A.; Tse H. L. A.; Mansour T. S.; Goodyear M. D.; Ravenscroft P.; Beels C. D. Diastereoselective Synthesis of the Potent Antiviral Agent (−)-2′-Deoxy-3′-thiacytidine and Its Enantiomer. J. Org. Chem. 1995, 60, 2621–2623. 10.1021/jo00113a050. - DOI
- Goodyear M. D.; Dwyer P. O.; Hill M. L.; Whitehead A. J.; Hornby R.; Hallet P.. Process for the Diastereoselective Synthesis of Nucleoside Analogues. US 6,051,709, 2000.
- Li J.-Z.; Gao L.-X.; Ding M.-X. The Chemical Resolution of Racemic cis-2-hydroxymethyl-5-(cytosine-1′-yl)-1,3-oxathiolane (BCH-189)—One Direct method to Obtain Lamivudine as Anti-HIV and Anti-HBV Agent. Synth. Commun. 2002, 32, 2355–2359. 10.1081/SCC-120006006. - DOI
- Goodyear M. D.; Hill M. L.; West J. P.; Whitehead A. J. Practical enantioselective synthesis of lamivudine (3TC) via a dynamic kinetic resolution. Tetrahedron Lett. 2005, 46, 8535–8538. 10.1016/j.tetlet.2005.10.002. - DOI
- Roy B. N.; Singh G. P.; Srivastava D.; Jadhav H. S.; Saini M. B.; Aher U. P. A Novel Method for Large-Scale Synthesis of Lamivudine through Cocrystal Formation of Racemic Lamivudine with (S)-(−)-1,1′-Bi(2-naphthol) [(S)-(BINOL)]. Org. Process Res. Dev. 2009, 13, 450–455. 10.1021/op800228h. - DOI
- Reddy B. P.; Reddy K. R.; Reddy R. R.; Reddy D. M.; Srinivas A. S.. Optical resolution of substituted 1,3-oxathiolane nucleosides. US 20110245497, 2011.
- Hu L.; Schaufelberger F.; Zhang Y.; Ramström O. Efficient asymmetric synthesis of lamivudine via enzymatic dynamic kinetic resolution. Chem. Commun. 2013, 49, 10376–10378. 10.1039/c3cc45551c. - DOI - PubMed
- Chen Y.; Zhang X.; Zheng G.; Gao S. Preparation of the Enantiomerically Enriched Precursor of Lamivudine (3TCTM) via Asymmetric Catalysis Mediated by Klebsiella Oxytoca. Process Biochem. 2019, 81, 77–84. 10.1016/j.procbio.2019.03.025. - DOI
- Zhang Y.; Sun Y.; Tang H.; Zhao Q.; Ren W.; Lv K.; Yang F.; Wang F.; Liu J. One-Pot Enzymatic Synthesis of Enantiopure 1,3-Oxathiolanes Using Trichosporon laibachii Lipase and the Kinetic Model. Org. Process Res. Dev. 2020, 24, 579–587. 10.1021/acs.oprd.0c00010. - DOI
- Snead D. R.; McQuade D. T.; Ahmad S.; Krack R.; Stringham R. W.; Burns J. M.; Abdiaj I.; Gopalsamuthiram V.; Nelson R. C.; Gupton B. F. An Economical Route to Lamivudine Featuring a Novel Strategy for Stereospecific Assembly. Org. Process Res. Dev. 2020, 10.1021/acs.oprd.0c00083. - DOI - PMC - PubMed
-
- Nair D. S.; Rai B. P.; Nizar H.; Meeran P. N.; Tewari N.. Process and intermediates for the preparation of substituted 1,3-oxathiolanes, especially lamivudine. US 20100311961, 2010.
- Tewari N.; Nair D. S.; Nizar H.; Meeran P. N.; Prasad M.. Process for the preparation of substituted 1,3-oxathiolanes. US 20100311970, 2010.
- Shankar R.; Vennapureddy R. R.; Sayyed A. P.; Ankaraju M. K.; Madasu S. B.; Vascuri J. R.; Meenakshisunderam S. Process for preparation of cis-nucleoside derivative. US 20110282046, 2011.
- Rama S.; Gorantla S. C. S.; Vadali L. R.; Inupakutika V. B. K. S.; Dasari S. R.; Mittapelly N.; Singh S. K.; Datta D.. Novel process for the preparation of cis-nucleoside derivative. US 20120295930, 2012.
- Roy B. N.; Singh G. P.; Srivastava D.; Aher U. P.; Patil S. U.. Stereoselective process for preparation of 1,3-oxathiolane nucleosides. US 20130296562, 2013.
- Caso M. F.; D’Alonzo D.; D’Errico S.; Palumbo G.; Guaragna A. Highly Stereoselective Synthesis of Lamivudine (3TC) and Emtricitabine (FTC) by a Novel N-Glycosidation Procedure. Org. Lett. 2015, 17, 2626–2629. 10.1021/acs.orglett.5b00982. - DOI - PubMed
- Mandala D.; Watts P. An Improved Synthesis of Lamivudine and Emtricitabine. Chemistry Select 2017, 2, 1102–1105. 10.1002/slct.201700052. - DOI
- Aher U. P.; Srivastava D.; Jadhav H. S.; Singh G. P.; B. S. J.; Shenoy G. G. Large-Scale Stereoselective Synthesis of 1,3-Oxathiolane Nucleoside, Lamivudine, via ZrCl4-Mediated N-Glycosylation. Org. Process Res. Dev. 2020, 24, 387–397. 10.1021/acs.oprd.9b00414. - DOI
-
- Smit V. A.; Zefirov N. S.; Bodrikov I. V.; Krimer M. Z. Episulfonium ions: myth and reality. Acc. Chem. Res. 1979, 12, 282–288. 10.1021/ar50140a003. - DOI
-
- Martin H. M.Manufacture of thio-acids and derivatives. US 2,413,361, 1945.
- Coons R. J.; Todaro C. A.. Process for producing α-mercapto-carboxylic acids. US 2,594,030, 1950.
LinkOut - more resources
Full Text Sources
