Guttiferone K Exerts the Anti-inflammatory Effect on Mycobacterium Tuberculosis- (H37Ra-) Infected Macrophages by Targeting the TLR/IRAK-1 Mediated Akt and NF- κ B Pathway
- PMID: 33100904
- PMCID: PMC7569438
- DOI: 10.1155/2020/8528901
Guttiferone K Exerts the Anti-inflammatory Effect on Mycobacterium Tuberculosis- (H37Ra-) Infected Macrophages by Targeting the TLR/IRAK-1 Mediated Akt and NF- κ B Pathway
Abstract
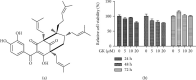
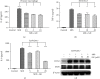
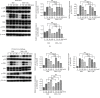
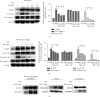
Mycobacterium tuberculosis (Mtb) remains a great threat to global health, killing more people than any other single infectious agent and causing uncontrollable inflammation in the host. Poorly controlled inflammatory processes can be deleterious and result in immune exhaustion. The current tuberculosis (TB) control is facing the challenge of drugs deficiency, especially in the context of increasingly multidrug resistant (MDR) TB. Under this circumstance, alternative host-directed therapy (HDT) emerges timely which can be exploited to improve the efficacy of TB treatment and disease prognosis by targeting the host. Here, we established the in vitro infection model of Mtb macrophages with H37Ra strain to seek effective anti-TB active agent. The present study showed that Guttiferone K, isolated from Garcinia yunnanensis, could significantly inhibit Mtb-induced inflammation in RAW264.7 and primary peritoneal macrophages. It was evidenced by the decreased production of inflammatory mediators, including interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2). Further studies with immunoblotting and immunofluorescence revealed that Guttiferone K obviously inhibits the nuclear factor-kappa B (NF-κB) both in RAW264.7 and primary peritoneal macrophages relying on the TLR/IRAK-1 pathway. Guttiferone K could also suppress the NLRP3 inflammasome activity and induce autophagy by inhibiting the protein kinase B (p-Akt) and mammalian target of rapamycin (mTOR) phosphorylation at Ser473 and Ser2448 in both cell lines. Thus, Guttiferone K possesses significant anti-inflammatory effect, alleviating Mtb-induced inflammation with an underlying mechanism that targeting on the TLR/IRAK-1 pathway and inhibiting the downstream NF-κB and Akt/mTOR signaling pathways. Together, Guttiferone K can be an anti-inflammatory agent candidate for the design of new adjunct HDT drugs fighting against tuberculosis.
Copyright © 2020 Qingwen Zhang et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Word Health Organization. Global Tuberculosis Report 2018. Geneva: WHO; 2018. https://www.who.int/tb/publications/global_report/en/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

