Synaptojanin2 Mutation Causes Progressive High-frequency Hearing Loss in Mice
- PMID: 33100973
- PMCID: PMC7546894
- DOI: 10.3389/fncel.2020.561857
Synaptojanin2 Mutation Causes Progressive High-frequency Hearing Loss in Mice
Abstract
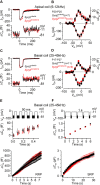
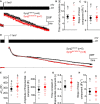
Progressive hearing loss is very common in the human population but we know little about the underlying molecular mechanisms. Synaptojanin2 (Synj2) has been reported to be involved, as a mouse mutation led to a progressive increase in auditory thresholds with age. Synaptojanin2 is a phosphatidylinositol (PI) phosphatase that removes the five-position phosphates from phosphoinositides, such as PIP2 and PIP3, and is a key enzyme in clathrin-mediated endocytosis. To investigate the mechanisms underlying progressive hearing loss, we have studied a different mutation of mouse Synj2 to look for any evidence of involvement of vesicle trafficking particularly affecting the synapses of sensory hair cells. Auditory brainstem responses (ABR) developed normally at first but started to decline between 3 and 4 weeks of age in Synj2tm1b mutants. At 6 weeks old, some evidence of outer hair cell (OHC) stereocilia fusion and degeneration was observed, but this was only seen in the extreme basal turn so cannot explain the raised ABR thresholds that correspond to more apical regions of the cochlear duct. We found no evidence of any defect in inner hair cell (IHC) exocytosis or endocytosis using single hair cell recordings, nor any sign of hair cell synaptic abnormalities. Endocochlear potentials (EP) were normal. The mechanism underlying progressive hearing loss in these mutants remains elusive, but our findings of raised distortion product otoacoustic emission (DPOAE) thresholds and signs of OHC degeneration both suggest an OHC origin for the hearing loss. Synaptojanin2 is not required for normal development of hearing but it is important for its maintenance.
Keywords: auditory function; exocytosis; hair cells; mouse mutant; progressive hearing loss; single hair cell recording; synaptojanin2.
Copyright © 2020 Martelletti, Ingham, Houston, Pass, Chen, Marcotti and Steel.
Figures









References
-
- Corns L. F., Bardhan T., Houston O., Olt J., Holley M. C., Masetto S., et al. (2014). “Functional development of hair cells in the mammalian inner ear,” in Development of Auditory and Vestibular Systems, eds Romand R., Varela-Nieto I. (New York, NY: Academic Press; ), 155–188.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

