Total Flavones of Abelmoschus manihot Remodels Gut Microbiota and Inhibits Microinflammation in Chronic Renal Failure Progression by Targeting Autophagy-Mediated Macrophage Polarization
- PMID: 33101025
- PMCID: PMC7554637
- DOI: 10.3389/fphar.2020.566611
Total Flavones of Abelmoschus manihot Remodels Gut Microbiota and Inhibits Microinflammation in Chronic Renal Failure Progression by Targeting Autophagy-Mediated Macrophage Polarization
Abstract
Background: Recently, progression of chronic renal failure (CRF) has been closely associated with gut microbiota dysbiosis and intestinal metabolite-derived microinflammation. In China, total flavones of Abelmoschus manihot (TFA), a component of Abelmoschus manihot, has been widely used to delay CRF progression in clinics for the past two decades. However, the overall therapeutic mechanisms remain obscure. In this study, we designed experiments to investigate the renoprotective effects of TFA in CRF progression and its underlying mechanisms involved in gut microbiota and microinflammation, compared with febuxostat (FEB), a potent non-purine selective inhibitor of xanthine oxidase.
Methods: In vivo, the CRF rat models were induced by uninephrectomy, potassium oxonate, and proinflammatory diet, and received either TFA suspension, FEB, or vehicle after modeling for 28 days. In vitro, the RAW 264.7 cells were exposed to lipopolysaccharide (LPS) with or without TFA or FEB. Changes in parameters related to renal injury, gut microbiota dysbiosis, gut-derived metabolites, and microinflammation were analyzed in vivo. Changes in macrophage polarization and autophagy and its related signaling were analyzed both in vivo and in vitro.
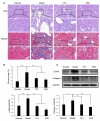
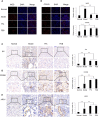
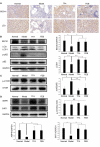
Results: For the modified CRF model rats, the administration of TFA and FEB improved renal injury, including renal dysfunction and renal tubulointerstitial lesions; remodeled gut microbiota dysbiosis, including decreased Bacteroidales and Lactobacillales and increased Erysipelotrichales; regulated gut-derived metabolites, including d-amino acid oxidase, serine racemase, d-serine, and l-serine; inhibited microinflammation, including interleukin 1β (IL1β), tumor necrosis factor-α, and nuclear factor-κB; and modulated macrophage polarization, including markers of M1/M2 macrophages. More importantly, TFA and FEB reversed the expression of beclin1 (BECN1) and phosphorylation of p62 protein and light chain 3 (LC3) conversion in the kidneys by activating the adenosine monophosphate-activated protein kinase-sirtuin 1 (AMPK-SIRT1) signaling. Further, TFA and FEB have similar effects on macrophage polarization and autophagy and its related signaling in vitro.
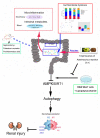
Conclusion: In this study, we demonstrated that TFA, similar to FEB, exerts its renoprotective effects partially by therapeutically remodeling gut microbiota dysbiosis and inhibiting intestinal metabolite-derived microinflammation. This is achieved by adjusting autophagy-mediated macrophage polarization through AMPK-SIRT1 signaling. These findings provide more accurate information on the role of TFA in delaying CRF progression.
Keywords: autophagy; chronic renal failure; gut microbiota; macrophage polarization; microinflammation; total flavones of Abelmoschus manihot.
Copyright © 2020 Tu, Fang, Sun, Liu, Liu, Wu, Yee, Yuan, Wang, Wan, Tang, Wan and Tang.
Figures





Similar articles
-
[Effects and mechanisms of total flavones of Abelmoschus manihot in inhibiting podocyte necroptosis and renal fibrosis in diabetic kidney disease].Zhongguo Zhong Yao Za Zhi. 2023 Aug;48(15):4137-4146. doi: 10.19540/j.cnki.cjcmm.20230417.701. Zhongguo Zhong Yao Za Zhi. 2023. PMID: 37802782 Chinese.
-
[Mechanism of total flavone of Abelmoschus manihot in treating ulcerative colitis and depression via intestinal flora-glycerophospholipid metabolism- macrophage polarization pathway].Zhongguo Zhong Yao Za Zhi. 2025 Mar;50(5):1286-1297. doi: 10.19540/j.cnki.cjcmm.20241111.701. Zhongguo Zhong Yao Za Zhi. 2025. PMID: 40350912 Chinese.
-
[Effects and mechanisms of total flavones of Abelmoschus manihot in attenuating diabetic tubulopathy by targeting endoplasmic reticulum stress-induced cell apoptosis].Zhongguo Zhong Yao Za Zhi. 2023 May;48(10):2657-2666. doi: 10.19540/j.cnki.cjcmm.20230216.702. Zhongguo Zhong Yao Za Zhi. 2023. PMID: 37282927 Chinese.
-
[Mechanisms and effects of Abelmoschus manihot preparations in treating chronic kidney disease].Zhongguo Zhong Yao Za Zhi. 2012 Aug;37(15):2252-6. Zhongguo Zhong Yao Za Zhi. 2012. PMID: 23189729 Review. Chinese.
-
[Pathomechanism and treatment of gut microbiota dysbiosis in chronic kidney disease and interventional effects of Chinese herbal medicine].Zhongguo Zhong Yao Za Zhi. 2017 Jul;42(13):2425-2432. doi: 10.19540/j.cnki.cjcmm.20170609.014. Zhongguo Zhong Yao Za Zhi. 2017. PMID: 28840678 Review. Chinese.
Cited by
-
Abelmoschus Manihot ameliorates the levels of circulating metabolites in diabetic nephropathy by modulating gut microbiota in non-obese diabetes mice.Microb Biotechnol. 2023 Apr;16(4):813-826. doi: 10.1111/1751-7915.14200. Epub 2022 Dec 30. Microb Biotechnol. 2023. PMID: 36583468 Free PMC article.
-
The dual regulatory effects of intestinal microorganisms and their metabolites in gouty arthritis pathogenesis: a balance between promotion and inhibition.Front Immunol. 2025 Jun 18;16:1591369. doi: 10.3389/fimmu.2025.1591369. eCollection 2025. Front Immunol. 2025. PMID: 40607437 Free PMC article. Review.
-
MALAT1 shuttled by extracellular vesicles promotes M1 polarization of macrophages to induce acute pancreatitis via miR-181a-5p/HMGB1 axis.J Cell Mol Med. 2021 Oct;25(19):9241-9254. doi: 10.1111/jcmm.16844. Epub 2021 Aug 27. J Cell Mol Med. 2021. PMID: 34448533 Free PMC article.
-
Prediction of delayed graft function by early salivary microbiota following kidney transplantation.Appl Microbiol Biotechnol. 2024 Jul 1;108(1):402. doi: 10.1007/s00253-024-13236-w. Appl Microbiol Biotechnol. 2024. PMID: 38951204 Free PMC article.
-
Quercetin Antagonizes Glucose Fluctuation Induced Renal Injury by Inhibiting Aerobic Glycolysis via HIF-1α/miR-210/ISCU/FeS Pathway.Front Med (Lausanne). 2021 Mar 4;8:656086. doi: 10.3389/fmed.2021.656086. eCollection 2021. Front Med (Lausanne). 2021. PMID: 33748166 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

