Progesterone Regulates Glucose Metabolism Through Glucose Transporter 1 to Promote Endometrial Receptivity
- PMID: 33101047
- PMCID: PMC7546771
- DOI: 10.3389/fphys.2020.543148
Progesterone Regulates Glucose Metabolism Through Glucose Transporter 1 to Promote Endometrial Receptivity
Abstract
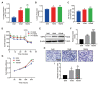
Successful embryo implantation requires receptive endometrium, which is conducive to the process of embryo recognition, adhesion, and invasion within a certain period of time and is inseparable from the dynamic interaction between 17β-estradiol (E2) and progesterone (P4). Proper glucose metabolism is critical for the profound physiological changes in the endometrium entering the receptive state. And glucose transporters (GLUTs) are responsible for intracellular uptake of glucose and are the first step in glucose metabolism. Prior literature has reported the presence of GLUTs in the endometrium. However, we still do not understand the specific mechanisms of this process. In this study, we identified the effect of P4 on glucose transporter 1 (GLUT1) using in vivo animal models and determined the regulation of glucose metabolism by P4 in cells. We highly suspect that this pregnancy failure may be due to reduced GLUT1-mediated glucose metabolism, resulting in a decrease in endometrial receptivity caused by an inadequate energy supply and synthesis of substrate. Here, we propose a possible mechanism to explain how embryo implantation is affected by P4 and glucose utilization under abnormal endometrial conditions.
Keywords: endometrial receptivity; glucose metabolism; glucose transporter 1; implantation; progesterone.
Copyright © 2020 Zhang, Qi, Wang, Sun, Li, Sui, Fan, Liu, Shang, Kong and Kong.
Figures




References
-
- Angulo C., Rauch M. C., Droppelmann A., Reyes A. M., Slebe J. C., Delgado-López F., et al. (1998). Hexose transporter expression and function in mammalian spermatozoa: cellular localization and transport of hexoses and vitamin C. J. Cell. Biochem. 71, 189–203. 10.1002/(SICI)1097-4644(19981101)71:2<189::AID-JCB5>3.0.CO;2-R, PMID: - DOI - PubMed
-
- Choi H. J., Chung T. W., Park M. J., Jung Y. S., Lee S. O., Kim K. J., et al. (2017). Water-extracted tubers of Cyperus rotundus L. enhance endometrial receptivity through leukemia inhibitory factor-mediated expression of integrin αVβ3 and αVβ5. J. Ethnopharmacol. 208, 16–23. 10.1016/j.jep.2017.06.051, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

