Extracellular Vesicle miRNAs in the Promotion of Cardiac Neovascularisation
- PMID: 33101061
- PMCID: PMC7546892
- DOI: 10.3389/fphys.2020.579892
Extracellular Vesicle miRNAs in the Promotion of Cardiac Neovascularisation
Abstract
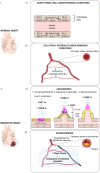
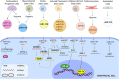
Cardiovascular disease (CVD) is the leading cause of mortality worldwide claiming almost 17. 9 million deaths annually. A primary cause is atherosclerosis within the coronary arteries, which restricts blood flow to the heart muscle resulting in myocardial infarction (MI) and cardiac cell death. Despite substantial progress in the management of coronary heart disease (CHD), there is still a significant number of patients developing chronic heart failure post-MI. Recent research has been focused on promoting neovascularisation post-MI with the ultimate goal being to reduce the extent of injury and improve function in the failing myocardium. Cardiac cell transplantation studies in pre-clinical models have shown improvement in cardiac function; nonetheless, poor retention of the cells has indicated a paracrine mechanism for the observed improvement. Cell communication in a paracrine manner is controlled by various mechanisms, including extracellular vesicles (EVs). EVs have emerged as novel regulators of intercellular communication, by transferring molecules able to influence molecular pathways in the recipient cell. Several studies have demonstrated the ability of EVs to stimulate angiogenesis by transferring microRNA (miRNA, miR) molecules to endothelial cells (ECs). In this review, we describe the process of neovascularisation and current developments in modulating neovascularisation in the heart using miRNAs and EV-bound miRNAs. Furthermore, we critically evaluate methods used in cell culture, EV isolation and administration.
Keywords: angiogenesis; cardiac; exosome (EXO); extracellular vesicles (EV); microRNA (miR); myocardial infarct; neovascularisation; regeneration.
Copyright © 2020 Kesidou, da Costa Martins, de Windt, Brittan, Beqqali and Baker.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

