Angiotensin Converting Enzyme 2 (ACE2) in Pregnancy: Preeclampsia and Small for Gestational Age
- PMID: 33101066
- PMCID: PMC7554608
- DOI: 10.3389/fphys.2020.590787
Angiotensin Converting Enzyme 2 (ACE2) in Pregnancy: Preeclampsia and Small for Gestational Age
Erratum in
-
Corrigendum: Angiotensin Converting Enzyme 2 (ACE2) in Pregnancy: Preeclampsia and Small for Gestational Age.Front Physiol. 2021 May 20;12:692761. doi: 10.3389/fphys.2021.692761. eCollection 2021. Front Physiol. 2021. PMID: 34093245 Free PMC article.
Abstract
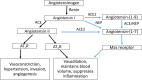
Introduction: An imbalance in angiotensin (Ang) peptides could contribute to the pathophysiology of preeclampsia (PE) and poor fetal growth.
Methods: We measured maternal plasma levels of Ang peptides and converting enzymes in non-pregnant women (n = 10), in normal pregnant women (n = 59), women delivering small for gestational age babies (SGA, n = 25) across gestation (13-36 weeks) and in women with PE (n = 14) in their third trimester.
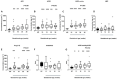
Results: Plasma ACE, ACE2, and Ang-(1-7) levels, and ACE2 activity were significantly higher in normal pregnant women compared with non-pregnant women; neprilysin (NEP) levels were not changed. In SGA pregnancies, ACE and ACE2 levels were higher in early-mid pregnancy compared with normal pregnant women. In women with PE, plasma ACE, ACE2, NEP, and Ang-(1-7) levels and ACE2 activity were lower than levels in normal pregnant women.
Conclusion: The higher plasma ACE2 levels and activity in pregnancy could be driving the higher Ang-(1-7) levels. The early gestation increases in ACE and ACE2 levels in SGA pregnancies highlights the possibility that these enzymes could be used as potential early biomarkers of poor fetal growth. In women with PE, the reduced ACE2 and NEP levels at term, could be contributing to the reduction in Ang-(1-7) levels. These findings suggest that dysfunctional relationships between two key enzymes in the circulating RAS are involved in the pathogenesis of PE and SGA. Since soluble ACE2 can prevent binding of the novel coronavirus, SARS-CoV-2, to membrane bound ACE2, the interplay between ACE2 and the coronavirus and its impact in pregnancy requires further investigation.
Keywords: angiotensin converting enzyme 2 (ACE2); angiotensin peptides; preeclampsia; pregnancy; small for gestational age.
Copyright © 2020 Tamanna, Clifton, Rae, van Helden, Lumbers and Pringle.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

