Temperate Bacteriophages (Prophages) in Pseudomonas aeruginosa Isolates Belonging to the International Cystic Fibrosis Clone (CC274)
- PMID: 33101229
- PMCID: PMC7546807
- DOI: 10.3389/fmicb.2020.556706
Temperate Bacteriophages (Prophages) in Pseudomonas aeruginosa Isolates Belonging to the International Cystic Fibrosis Clone (CC274)
Abstract
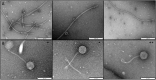
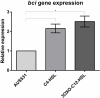
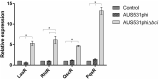
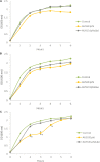
Bacteriophages are important in bacterial ecology and evolution. Pseudomonas aeruginosa is the most prevalent bacterial pathogen in chronic bronchopulmonary infection in cystic fibrosis (CF). In this study, we used bioinformatics, microbiological and microscopy techniques to analyze the bacteriophages present in 24 P. aeruginosa isolates belonging to the international CF clone (ST274-CC274). Interestingly, we detected the presence of five members of the Inoviridae family of prophages (Pf1, Pf4, Pf5, Pf6, Pf7), which have previously been observed in P. aeruginosa. In addition, we identified a new filamentous prophage, designated Pf8, in the P. aeruginosa AUS411.500 isolate belonging to the international CF clone. We detected only one prophage, never previously described, from the family Siphoviridiae (with 66 proteins and displaying homology with PHAGE_Pseudo_phi297_NC_016762). This prophage was isolated from the P. aeruginosa AUS531 isolate carrying a new gene which is implicated in the phage infection ability, named Bacteriophage Control Infection (bci). We characterized the role of the Bci protein in bacteriophage infection and in regulating the host Quorum Sensing (QS) system, motility and biofilm and pyocyanin production in the P. aeruginosa isogenic mutant AUS531Δbci isolate. The findings may be relevant for the identification of targets in the development of new strategies to control P. aeruginosa infections, particularly in CF patients.
Keywords: CC274 clone; Pseudomonas; cystic fibrosis; inovirus; prophages; siphovirus.
Copyright © 2020 Ambroa, Blasco, López-Causapé, Trastoy, Fernandez-García, Bleriot, Ponce-Alonso, Pacios, López, Cantón, Kidd, Bou, Oliver and Tomás.
Figures

Similar articles
-
Prevalence of Pf1-like (pro)phage genetic elements among Pseudomonas aeruginosa isolates.Virology. 2015 Sep;483:64-71. doi: 10.1016/j.virol.2015.04.008. Epub 2015 May 15. Virology. 2015. PMID: 25965796
-
Analysis of Pseudomonas aeruginosa Isolates from Patients with Cystic Fibrosis Revealed Novel Groups of Filamentous Bacteriophages.Viruses. 2023 Nov 5;15(11):2215. doi: 10.3390/v15112215. Viruses. 2023. PMID: 38005892 Free PMC article.
-
Characterization of the integrated filamentous phage Pf5 and its involvement in small-colony formation.Microbiology (Reading). 2007 Jun;153(Pt 6):1790-1798. doi: 10.1099/mic.0.2006/003533-0. Microbiology (Reading). 2007. PMID: 17526836 Free PMC article.
-
The role of quorum sensing in chronic cystic fibrosis Pseudomonas aeruginosa infections.FEMS Microbiol Lett. 2009 Jan;290(1):1-9. doi: 10.1111/j.1574-6968.2008.01394.x. Epub 2008 Oct 29. FEMS Microbiol Lett. 2009. PMID: 19016870 Review.
-
Pf Bacteriophage and Their Impact on Pseudomonas Virulence, Mammalian Immunity, and Chronic Infections.Front Immunol. 2020 Feb 21;11:244. doi: 10.3389/fimmu.2020.00244. eCollection 2020. Front Immunol. 2020. PMID: 32153575 Free PMC article. Review.
Cited by
-
Exploring the occurrence of Pseudomonas aeruginosa and comprehensive whole genome analysis of the bcsir_p4_s20 strain from municipal wastewater in Chattogram.World J Microbiol Biotechnol. 2025 Mar 28;41(4):112. doi: 10.1007/s11274-025-04328-4. World J Microbiol Biotechnol. 2025. PMID: 40148700
-
Shotgun Metagenomic Sequencing Analysis as a Diagnostic Strategy for Patients with Lower Respiratory Tract Infections.Microorganisms. 2025 Jun 9;13(6):1338. doi: 10.3390/microorganisms13061338. Microorganisms. 2025. PMID: 40572226 Free PMC article.
-
Phage-mediated resolution of genetic conflict alters the evolutionary trajectory of Pseudomonas aeruginosa lysogens.mSystems. 2024 Sep 17;9(9):e0080124. doi: 10.1128/msystems.00801-24. Epub 2024 Aug 21. mSystems. 2024. PMID: 39166874 Free PMC article.
-
Genome-Based Analysis of Virulence Factors and Biofilm Formation in Novel P. aeruginosa Strains Isolated from Household Appliances.Microorganisms. 2022 Dec 19;10(12):2508. doi: 10.3390/microorganisms10122508. Microorganisms. 2022. PMID: 36557761 Free PMC article.
-
Temperate phage-antibiotic synergy is widespread-extending to Pseudomonas-but varies by phage, host strain, and antibiotic pairing.mBio. 2025 Feb 5;16(2):e0255924. doi: 10.1128/mbio.02559-24. Epub 2024 Dec 20. mBio. 2025. PMID: 39704503 Free PMC article.
References
-
- Alič Š, Naglič T., Tušek-Žnidariè M., Ravnikar M., Raèki N., Peterka M., et al. (2017). Newly Isolated Bacteriophages from the Podoviridae, Siphoviridae, and Myoviridae Families Have Variable Effects on Putative Novel Dickeya spp. Front Microbiol 8:1870. 10.3389/fmicb.2017.01870 - DOI - PMC - PubMed
-
- Cady K. C., White A. S., Hammond J. H., Abendroth M. D., Karthikeyan R. S., Lalitha P., et al. (2011). Prevalence, conservation and functional analysis of Yersinia and Escherichia CRISPR regions in clinical Pseudomonas aeruginosa isolates. Microbiology. Feb 157 430–437. 10.1099/mic.0.045732-0 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

