Glucose-Mediated Protein Arginine Phosphorylation/Dephosphorylation Regulates ylxR Encoding Nucleoid-Associated Protein and Cell Growth in Bacillus subtilis
- PMID: 33101263
- PMCID: PMC7546277
- DOI: 10.3389/fmicb.2020.590828
Glucose-Mediated Protein Arginine Phosphorylation/Dephosphorylation Regulates ylxR Encoding Nucleoid-Associated Protein and Cell Growth in Bacillus subtilis
Abstract
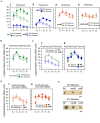
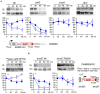
Glucose is the most favorable carbon source for many bacteria, and these bacteria have several glucose-responsive networks. We proposed new glucose responsive system, which includes protein acetylation and probable translation control through TsaEBD, which is a tRNA modification enzyme required for the synthesis of threonylcarbamoyl adenosine (t6A)-tRNA. The system also includes nucleoid-associated protein YlxR, regulating more than 400 genes including many metabolic genes and the ylxR-containing operon driven by the PylxS promoter is induced by glucose. Thus, transposon mutagenesis was performed for searching regulatory factors for PylxS expression. As a result, ywlE was identified. The McsB kinase phosphorylates arginine (Arg) residues of proteins and the YwlE phosphatase counteracts against McsB through Arg-dephosphorylation. Phosphorylated Arg has been known to function as a tag for ClpCP-dependent protein degradation. The previous analysis identified TsaD as an Arg-phosphorylated protein. Our results showed that the McsB/YwlE system regulates PylxS expression through ClpCP-mediated protein degradation of TsaD. In addition, we observed that glucose induced ywlE expression and repressed mcsB expression. It was concluded that these phenomena would cause glucose induction (GI) of PylxS, based on the Western blot analyses of TsaD-FLAG. These observations and the previous those that many glycolytic enzymes are Arg-phosphorylated suggested that the McsB/YwlE system might be involved in cell growth in glucose-containing medium. We observed that the disruption of mcsB and ywlE resulted in an increase of cell mass and delayed growth, respectively, in semi-synthetic medium. These results provide us broader insights to the physiological roles of the McsB/YwlE system and protein Arg-phosphorylation.
Keywords: ClpCP protease; YwlE phosphatase; glucose response; glycolysis; protein acetylation.
Copyright © 2020 Ogura.
Figures




References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

