Innate Immune Responses to Acute Viral Infection During Pregnancy
- PMID: 33101294
- PMCID: PMC7556209
- DOI: 10.3389/fimmu.2020.572567
Innate Immune Responses to Acute Viral Infection During Pregnancy
Abstract
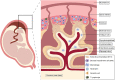
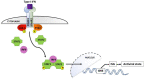
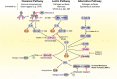
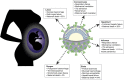
Immunological adaptations in pregnancy allow maternal tolerance of the semi-allogeneic fetus but also increase maternal susceptibility to infection. At implantation, the endometrial stroma, glands, arteries and immune cells undergo anatomical and functional transformation to create the decidua, the specialized secretory endometrium of pregnancy. The maternal decidua and the invading fetal trophoblast constitute a dynamic junction that facilitates a complex immunological dialogue between the two. The decidual and peripheral immune systems together assume a pivotal role in regulating the critical balance between tolerance and defense against infection. Throughout pregnancy, this equilibrium is repeatedly subjected to microbial challenge. Acute viral infection in pregnancy is associated with a wide spectrum of adverse consequences for both mother and fetus. Vertical transmission from mother to fetus can cause developmental anomalies, growth restriction, preterm birth and stillbirth, while the mother is predisposed to heightened morbidity and maternal death. A rapid, effective response to invasive pathogens is therefore essential in order to avoid overwhelming maternal infection and consequent fetal compromise. This sentinel response is mediated by the innate immune system: a heritable, highly evolutionarily conserved system comprising physical barriers, antimicrobial peptides (AMP) and a variety of immune cells-principally neutrophils, macrophages, dendritic cells, and natural killer cells-which express pattern-receptors that detect invariant molecular signatures unique to pathogenic micro-organisms. Recognition of these signatures during acute infection triggers signaling cascades that enhance antimicrobial properties such as phagocytosis, secretion of pro-inflammatory cytokines and activation of the complement system. As well as coordinating the initial immune response, macrophages and dendritic cells present microbial antigens to lymphocytes, initiating and influencing the development of specific, long-lasting adaptive immunity. Despite extensive progress in unraveling the immunological adaptations of pregnancy, pregnant women remain particularly susceptible to certain acute viral infections and continue to experience mortality rates equivalent to those observed in pandemics several decades ago. Here, we focus specifically on the pregnancy-induced vulnerabilities in innate immunity that contribute to the disproportionately high maternal mortality observed in the following acute viral infections: Lassa fever, Ebola virus disease (EVD), dengue fever, hepatitis E, influenza, and novel coronavirus infections.
Keywords: Ebola virus; Lassa virus; dengue virus; emerging coronavirus; hepatitis E; influenza virus; innate antiviral immunity; pregnancy.
Copyright © 2020 Cornish, Filipovic, Åsenius, Williams and McDonnell.
Figures

References
-
- Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol. (1953) 44:320–38.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

