Comparison of Bead-Based Fluorescence Versus Planar Electrochemiluminescence Multiplex Immunoassays for Measuring Cytokines in Human Plasma
- PMID: 33101295
- PMCID: PMC7546899
- DOI: 10.3389/fimmu.2020.572634
Comparison of Bead-Based Fluorescence Versus Planar Electrochemiluminescence Multiplex Immunoassays for Measuring Cytokines in Human Plasma
Abstract
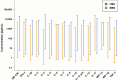
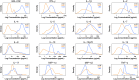
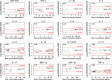
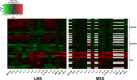
This study compared two 96-well multiplex immunoassay platforms for analytical performance in assessing cytokine concentrations in standards, quality controls and human plasma samples (n = 62), and evaluated assay time requirements. Assays included a bead-based fluorescence MILLIPLEX® assay/Luminex fluorescence platform (LMX) and three kits from Meso Scale Discovery (MSD) in planar electrochemiluminescence format. The LMX kit evaluated 21 cytokines and the MSD kits evaluated 10 cytokines each, with 16 overlapping cytokines between platforms. Both assays provided good reproducibility in standard curves for all analytes. Interassay CVs of shared analytes showed average kit quality control CVs ranging 1.9-18.2% for LMX and 2.4-13.9% for MSD. The MSD platform had lower LLoQs than LMX for 14/16 shared cytokines. For IL-17, the LLoQ was lower with LMX than MSD, and the LLoQs for IL-6 were similar. Although MSD calibration curves indicated lower LLoQs for most of those analytes, many more cytokines in human plasma samples were not detected by MSD than by LMX. The ULoQs were higher in LMX versus MSD assays for 13/16 shared analytes, lower than MSD for IL-17, and equivalent between assays for IL-6 and MIP-1α. Bland-Altman plots indicated that MSD classified 13/16 shared analytes as concentrations lower than by LMX. Time and motion analysis indicated that total mean assay times were 20 h 28 m and 21 h 33 m for LMX and MSD, respectively, including an overnight (17 h) incubation. The MSD assays employed a manufacturer-approved overnight incubation instead of the standard 2-h incubation, which kit instructions suggest might increase detection sensitivity. Hands-on labor time averaged 1 h 37 m for LMX and 2 h 33 m for MSD. In summary, assay selection factors should include selection of specific markers of interest, time and cost considerations, and anticipated cytokine concentrations in prospective samples.
Keywords: Luminex; Meso Scale Discovery; Millipore; chemokine; cytokine; dynamic range; lower limit of quantification; multiplex immunoassay.
Copyright © 2020 Günther, Becker, Göpfert, Joos and Schneiderhan-Marra.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

