4C3 Human Monoclonal Antibody: A Proof of Concept for Non-pathogenic Proteinase 3 Anti-neutrophil Cytoplasmic Antibodies in Granulomatosis With Polyangiitis
- PMID: 33101296
- PMCID: PMC7546423
- DOI: 10.3389/fimmu.2020.573040
4C3 Human Monoclonal Antibody: A Proof of Concept for Non-pathogenic Proteinase 3 Anti-neutrophil Cytoplasmic Antibodies in Granulomatosis With Polyangiitis
Abstract
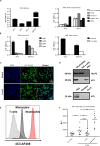
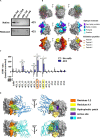
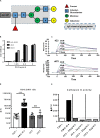
Granulomatosis with polyangiitis (GPA) is a severe autoimmune vasculitis associated with the presence of anti-neutrophil cytoplasmic antibodies (ANCA) mainly targeting proteinase 3 (PR3), a neutrophilic serine proteinase. PR3-ANCA binding to membrane-bound PR3 on neutrophils induce their auto-immune activation responsible for vascular lesions. However, the correlation between PR3-ANCA level and disease activity remains inconsistent, suggesting the existence of non-pathogenic PR3-ANCA. In order to prove their existence, we immortalized B lymphocytes from blood samples of GPA patients in remission having persistent PR3-ANCA to isolate non-activating PR3-ANCA. We obtained for the first time a non-activating human IgG1κ anti-PR3 monoclonal antibody (mAb) named 4C3. This new mAb binds soluble PR3 with a high affinity and membrane-bound PR3 on an epitope close to the PR3 hydrophobic patch and in the vicinity of the active site. 4C3 is able to bind FcγRIIA and FcγRIIIB and has a G2F glycosylation profile on asparagine 297. 4C3 did not induce activation of neutrophils and could inhibit human polyclonal PR3-ANCA-induced activation suggesting that 4C3 is non-pathogenic. This characteristic relies on the recognized epitope on PR3 rather than to the Fc portion properties. The existence of non-pathogenic PR3-ANCA, which do not activate neutrophils, could explain the persistence of high PR3-ANCA levels in some GPA patients in remission and why PR3-ANCA would not predict relapse. Finally, these results offer promising perspectives particularly regarding the understanding of PR3-ANCA pathogenicity and the development of new diagnostic and therapeutic strategies in GPA.
Keywords: anti-neutrophil cytoplasmic antibodies; epitope; granulomatosis with polyangiitis; human neutrophils; proteinase 3.
Copyright © 2020 Granel, Lemoine, Morello, Gallais, Mariot, Drapeau, Musnier, Poupon, Pugnière, Seren, Nouar, Gouilleux-Gruart, Watier, Korkmaz and Hoarau.
Figures

References
-
- Korkmaz B, Lesner A, Letast S, Mahdi YK, Jourdan M-L, Dallet-Choisy S, et al. Neutrophil proteinase 3 and dipeptidyl peptidase I (cathepsin C) as pharmacological targets in granulomatosis with polyangiitis (Wegener granulomatosis). Semin Immunopathol. (2013) 35:411–21. 10.1007/s00281-013-0362-z - DOI - PubMed
-
- Damoiseaux J, Csernok E, Rasmussen N, Moosig F, van Paassen P, Baslund B, et al. Detection of antineutrophil cytoplasmic antibodies (ANCAs): a multicentre European Vasculitis Study Group (EUVAS) evaluation of the value of indirect immunofluorescence (IIF) versus antigen-specific immunoassays. Ann Rheum Dis. (2017) 76:647–53. 10.1136/annrheumdis-2016-209507 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

