Japanese Encephalitis Virus Vaccination Elicits Cross-Reactive HLA-Class I-Restricted CD8 T Cell Response Against Zika Virus Infection
- PMID: 33101303
- PMCID: PMC7546338
- DOI: 10.3389/fimmu.2020.577546
Japanese Encephalitis Virus Vaccination Elicits Cross-Reactive HLA-Class I-Restricted CD8 T Cell Response Against Zika Virus Infection
Abstract
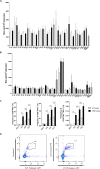
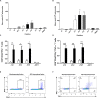
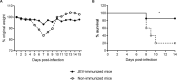
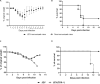
Japanese encephalitis virus (JEV) exposure or vaccination could elicit cross-reactive CD8 T cell immunity against heterologous flaviviruses in humans. In addition, cross-reactive CD8 T cells induced by dengue virus (DENV) have been shown to play a protective role against Zika virus (ZIKV). However, how JEV exposure or vaccination affects ZIKV infection in humans remains unclear. In this report, epitope prediction algorithms were used to predict the cross-reactive CD8 T cell epitope restricted to human HLA between JEV and ZIKV. We found that these predicted CD8 T cell epitopes are immunogenic and cross-reactive in humanized HLA transgenic mice. Moreover, JEV vaccine immunization provided cross-protection against ZIKV infection. Furthermore, CD8 T cells were involved in the protection against ZKIV infection in vivo. Our results have an important clinical implication that vaccination with JEV SA14-14-2 may provide protection against ZIKV infection in humans.
Keywords: CD8 T cells; HLA-A2 transgenic mice; JEV; Zika; cross-reactive; epitope; heterologous immunity.
Copyright © 2020 Tarbe, Dong, Hu, Xu, Sun, Grayo, Chen, Qin, Zhao, Liu, Li and Leng.
Figures

Similar articles
-
T cell immunity rather than antibody mediates cross-protection against Zika virus infection conferred by a live attenuated Japanese encephalitis SA14-14-2 vaccine.Appl Microbiol Biotechnol. 2020 Aug;104(15):6779-6789. doi: 10.1007/s00253-020-10710-z. Epub 2020 Jun 15. Appl Microbiol Biotechnol. 2020. PMID: 32556415 Free PMC article.
-
Identification of Zika virus epitopes reveals immunodominant and protective roles for dengue virus cross-reactive CD8+ T cells.Nat Microbiol. 2017 Mar 13;2:17036. doi: 10.1038/nmicrobiol.2017.36. Nat Microbiol. 2017. PMID: 28288094 Free PMC article.
-
Dengue virus-reactive CD8+ T cells mediate cross-protection against subsequent Zika virus challenge.Nat Commun. 2017 Nov 13;8(1):1459. doi: 10.1038/s41467-017-01669-z. Nat Commun. 2017. PMID: 29129917 Free PMC article.
-
Cross-Reactive T Cell Immunity to Dengue and Zika Viruses: New Insights Into Vaccine Development.Front Immunol. 2019 Jun 11;10:1316. doi: 10.3389/fimmu.2019.01316. eCollection 2019. Front Immunol. 2019. PMID: 31244855 Free PMC article. Review.
-
CD4+ and CD8+ T-cell immunity to Dengue - lessons for the study of Zika virus.Immunology. 2017 Feb;150(2):146-154. doi: 10.1111/imm.12681. Epub 2016 Nov 27. Immunology. 2017. PMID: 27763656 Free PMC article. Review.
Cited by
-
Cross-Reactive Immunity among Five Medically Important Mosquito-Borne Flaviviruses Related to Human Diseases.Viruses. 2022 Jun 2;14(6):1213. doi: 10.3390/v14061213. Viruses. 2022. PMID: 35746683 Free PMC article. Review.
-
Pre-existing Immunity to Japanese Encephalitis Virus Alters CD4 T Cell Responses to Zika Virus Inactivated Vaccine.Front Immunol. 2021 Feb 24;12:640190. doi: 10.3389/fimmu.2021.640190. eCollection 2021. Front Immunol. 2021. PMID: 33717194 Free PMC article. Clinical Trial.
-
Identification of immunodominant T cell epitopes induced by natural Zika virus infection.Front Immunol. 2023 Aug 29;14:1247876. doi: 10.3389/fimmu.2023.1247876. eCollection 2023. Front Immunol. 2023. PMID: 37705976 Free PMC article.
-
Acute neurologic emerging flaviviruses.Ther Adv Infect Dis. 2022 Jun 13;9:20499361221102664. doi: 10.1177/20499361221102664. eCollection 2022 Jan-Dec. Ther Adv Infect Dis. 2022. PMID: 35719177 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

