Emerging Concepts and Technologies in Vaccine Development
- PMID: 33101309
- PMCID: PMC7554600
- DOI: 10.3389/fimmu.2020.583077
Emerging Concepts and Technologies in Vaccine Development
Abstract
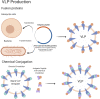
Despite the success of vaccination to greatly mitigate or eliminate threat of diseases caused by pathogens, there are still known diseases and emerging pathogens for which the development of successful vaccines against them is inherently difficult. In addition, vaccine development for people with compromised immunity and other pre-existing medical conditions has remained a major challenge. Besides the traditional inactivated or live attenuated, virus-vectored and subunit vaccines, emerging non-viral vaccine technologies, such as viral-like particle and nanoparticle vaccines, DNA/RNA vaccines, and rational vaccine design, offer innovative approaches to address existing challenges of vaccine development. They have also significantly advanced our understanding of vaccine immunology and can guide future vaccine development for many diseases, including rapidly emerging infectious diseases, such as COVID-19, and diseases that have not traditionally been addressed by vaccination, such as cancers and substance abuse. This review provides an integrative discussion of new non-viral vaccine development technologies and their use to address the most fundamental and ongoing challenges of vaccine development.
Keywords: COVID19; cancer vaccines; infectious disease; nanoparticle vaccines; non-viral DNA-RNA vaccines; noncommunicable disease; substance abuse; virus-like particle vaccines.
Copyright © 2020 Brisse, Vrba, Kirk, Liang and Ly.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

