Maresin-1 and Resolvin E1 Promote Regenerative Properties of Periodontal Ligament Stem Cells Under Inflammatory Conditions
- PMID: 33101318
- PMCID: PMC7546375
- DOI: 10.3389/fimmu.2020.585530
Maresin-1 and Resolvin E1 Promote Regenerative Properties of Periodontal Ligament Stem Cells Under Inflammatory Conditions
Abstract
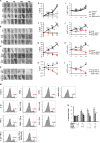
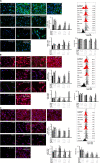
Maresin-1 (MaR1) and Resolvin E1 (RvE1) are specialized pro-resolving lipid mediators (SPMs) that regulate inflammatory processes. We have previously demonstrated the hard and soft tissue regenerative capacity of RvE1 in an in vivo model of the periodontal disease characterized by inflammatory tissue destruction. Regeneration of periodontal tissues requires a well-orchestrated process mediated by periodontal ligament stem cells. However, limited data are available on how SPMs can regulate the regenerative properties of human periodontal ligament stem cells (hPDLSCs) under inflammatory conditions. Thus, we measured the impact of MaR1 and RvE1 in an in vitro model of hPDLSC under stimulation with IL-1β and TNF-α by evaluating pluripotency, migration, viability/cell death, periodontal ligament markers (α-smooth muscle actin, tenomodulin, and periostin), cementogenic-osteogenic differentiation, and phosphoproteomic perturbations. The data showed that the pro-inflammatory milieu suppresses pluripotency, viability, and migration of hPDLSCs; MaR1 and RvE1 both restored regenerative capacity by increasing hPDLSC viability, accelerating wound healing/migration, and up-regulating periodontal ligament markers and cementogenic-osteogenic differentiation. Protein phosphorylation perturbations were associated with the SPM-induced regenerative capacity of hPDLSCs. Together, these results demonstrate that MaR1 and RvE1 restore or improve the regenerative properties of highly specialized stem cells when inflammation is present and offer opportunities for direct pharmacologic treatment of lost tissue integrity.
Keywords: Maresin 1; Omega 3 (n–3) polyunsaturated fatty acids; Resolvin E1; inflammation; stem cells.
Copyright © 2020 Albuquerque-Souza, Schulte, Chen, Hardt, Hasturk, Van Dyke, Holzhausen and Kantarci.
Figures

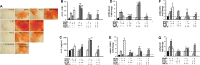


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

