Redox Status, JA and ET Signaling Pathway Regulating Responses to Botrytis cinerea Infection Between the Resistant Cucumber Genotype and Its Susceptible Mutant
- PMID: 33101327
- PMCID: PMC7546314
- DOI: 10.3389/fpls.2020.559070
Redox Status, JA and ET Signaling Pathway Regulating Responses to Botrytis cinerea Infection Between the Resistant Cucumber Genotype and Its Susceptible Mutant
Abstract
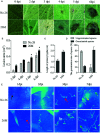
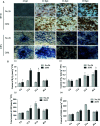
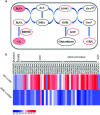
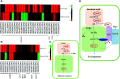
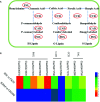
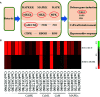
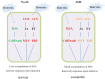
Botrytis cinerea is an important necrotrophic fungal pathogen with a broad host range and the ability to causing great economic losses in cucumber. However, the resistance mechanism against this pathogen in cucumber was not well understood. In this study, the microscopic observation of the spore growth, redox status measurements and transcriptome analysis were carried out after Botrytis cinerea infection in the resistant genotype No.26 and its susceptible mutant 26M. Results revealed shorter hypha, lower rate of spore germination, less acceleration of H2O2, O2 -, and lower total glutathione content (GSH+GSSG) in No.26 than that in 26M, which were identified by the staining result of DAB and NBT. Transcriptome data showed that after pathogen infection, a total of 3901 and 789 different expression genes (DEGs) were identified in No.26 and 26M respectively. These DEGs were highly enriched in redox regulation pathway, hormone signaling pathway and plant-pathogen interaction pathway. The glutathione S-transferase genes, putative peroxidase gene, and NADPH oxidase were up-regulated in No.26 whereas these genes changed little in 26M after Botrytis cinerea infection. Jasmonic acid and ethylene biosynthesis and signaling pathways were distinctively activated in No.26 comparing with 26M upon infection. Much more plant defense related genes including mitogen-activated protein kinases, calmodulin, calmodulin-like protein, calcium-dependent protein kinase, and WRKY transcription factor were induced in No.26 than 26M after pathogen infection. Finally, a model was established which elucidated the resistance difference between resistant cucumber genotype and susceptible mutant after B. cinerea infection.
Keywords: B. cinerea; cucumber; redox status; resistant; signaling pathway.
Copyright © 2020 Yang, Wang, Chen, Zhou, Xue, Abid and Chen.
Figures

References
LinkOut - more resources
Full Text Sources

