Small molecule 2,3-DCPE induces S phase arrest by activating the ATM/ATR-Chk1-Cdc25A signaling pathway in DLD-1 colon cancer cells
- PMID: 33101488
- PMCID: PMC7576987
- DOI: 10.3892/ol.2020.12157
Small molecule 2,3-DCPE induces S phase arrest by activating the ATM/ATR-Chk1-Cdc25A signaling pathway in DLD-1 colon cancer cells
Abstract
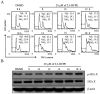
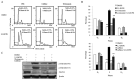
In our previous study, it was reported that 2[[3-(2,3-dichlorophenoxy)propyl]amino]ethanol (2,3-DCPE) induces apoptosis and cell cycle arrest. The current study aimed to investigate the molecular mechanism involved in 2,3-DCPE-induced S phase arrest. The results demonstrated that 2,3-DCPE upregulated phosphorylated (p-)H2A histone family member X, a biomarker of DNA damage, in the DLD-1 colon cancer cell line. Western blotting revealed that 2,3-DCPE increased the checkpoint kinase (Chk)1 (Ser317 and Ser345) level and decreased the expression of M-phase inducer phosphatase 1 (Cdc25A) in a time-dependent manner. Subsequently, the results demonstrated that the ataxia-telangiectasia mutated (ATM) and ataxia-telangiectasia and Rad3-related (ATR) inhibitors wortmannin and caffeine had no effect on the cell cycle; however, the inhibitors partially abrogated 2,3-DCPE-induced S phase arrest. Flow cytometry assays revealed that caffeine (2 mM) reduced the proportion of S phase cells from 83 to 39.6% and that wortmannin (500 nM) reduced the proportion of S phase cells from 83 to 48.2%. Furthermore, wortmannin and caffeine inhibited the 2,3-DCPE-mediated phosphorylation of Chk1 and the degradation of Cdc25A. However, these ATM/ATR inhibitors had limited effect on 2,3-DCPE-induced apoptosis. Taken together, the data of the current study indicated that 2,3-DCPE caused DNA damage in colon cancer cells and that 2,3-DCPE-induced S phase arrest was associated with the activation of the ATM/ATR-Chk1-Cdc25A pathway.
Keywords: 2,3-DCPE; ATM/ATR pathway; DNA damage; S phase arrest; colorectal cancer.
Copyright: © Bai et al.
Figures


References
-
- Vidimar V, Licona C, Cerón-Camacho R, Guerin E, Coliat P, Venkatasamy A, Ali M, Guenot D, Le Lagadec R, Jung AC, et al. A redox ruthenium compound directly targets PHD2 and inhibits the HIF1 pathway to reduce tumor angiogenesis independently of p53. Cancer Lett 440–441. 2019:145–155. doi: 10.1016/j.canlet.2018.09.029. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
