Potent antitumor activity of cepharanthine against triple-negative breast cancer spheroids compared with tetrandrine
- PMID: 33101499
- PMCID: PMC7577078
- DOI: 10.3892/ol.2020.12191
Potent antitumor activity of cepharanthine against triple-negative breast cancer spheroids compared with tetrandrine
Abstract
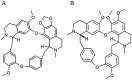
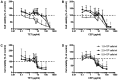
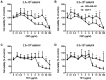
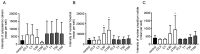
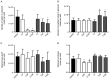
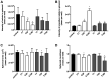
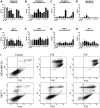
Cepharanthine (CEP) is a bis-bynzelisoquinoline alkaloid from the same class as the anticancer agent tetrandrine (TET). However, the effects of CEP against breast cancer have not been extensively studied, despite its long therapeutic history with low toxicity against other types of cancer. 3D culture systems more accurately mimic the human body and address the limitations of determining drug effectiveness compared with 2D culture systems. In the present study, the antitumor activities of TET and CEP were compared in 3D culture systems in triple-negative breast cancer (TNBC) MDA-MB-231 and estrogen receptor-positive breast cancer MCF-7 cell lines. Cell viability, apoptosis and cytotoxicity assays were performed to determine the total number of live or dead cells, the IC50 values, the number of apoptotic cells and spheroid roundness. Viability suppression of MDA-MB-231 cells was significantly greater with both TET and CEP compared with that of MCF-7 cells, and the roundness of MDA-MB-231 spheroids treated with CEP was decreased significantly compared with that of spheroid treated with TET. Cytoplasmic shrinkage in each cell line significantly increased with the treatment of TET compared with the control; however, this effect was stronger with CEP. The ratio of dead/live cells in each cell line treated with TET and CEP increased in a dose-dependent manner. Overall, the present study demonstrated that CEP had greater cell toxicity in 3D spheroids of breast cancer cells compared with TET, suggesting that CEP may have a stronger antitumor activity on TNBC spheroids compared with TET.
Keywords: 3D culture; CEP; TET; antitumor; breast cancer; spheroid.
Copyright: © Kiyomi et al.
Figures

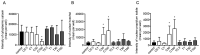




Similar articles
-
Tetrandrine and cepharanthine induce apoptosis through caspase cascade regulation, cell cycle arrest, MAPK activation and PI3K/Akt/mTOR signal modification in glucocorticoid resistant human leukemia Jurkat T cells.Chem Biol Interact. 2019 Sep 1;310:108726. doi: 10.1016/j.cbi.2019.108726. Epub 2019 Jun 28. Chem Biol Interact. 2019. PMID: 31255635
-
Characterization of Triple-Negative Breast Cancer MDA-MB-231 Cell Spheroid Model.Onco Targets Ther. 2020 Jun 11;13:5395-5405. doi: 10.2147/OTT.S249756. eCollection 2020. Onco Targets Ther. 2020. PMID: 32606757 Free PMC article.
-
The effects of CEP-37440, an inhibitor of focal adhesion kinase, in vitro and in vivo on inflammatory breast cancer cells.Breast Cancer Res. 2016 Mar 24;18(1):37. doi: 10.1186/s13058-016-0694-4. Breast Cancer Res. 2016. PMID: 27009091 Free PMC article.
-
Stilbene-rich extract increases the cytotoxic effects of paclitaxel in hormone receptor-positive and triple-negative breast cancer spheroids.Phytomed Plus. 2024 Aug;4(3):100578. doi: 10.1016/j.phyplu.2024.100578. Epub 2024 May 13. Phytomed Plus. 2024. PMID: 39574482 Free PMC article.
-
Current status and future challenges in extraction, purification and identification of Cepharanthine (a potential drug against COVID-19).Sep Purif Technol. 2023 Mar 15;309:123038. doi: 10.1016/j.seppur.2022.123038. Epub 2022 Dec 29. Sep Purif Technol. 2023. PMID: 36593875 Free PMC article. Review.
Cited by
-
Development of Breast Cancer Spheroids to Evaluate Cytotoxic Response to an Anticancer Peptide.Pharmaceutics. 2021 Nov 4;13(11):1863. doi: 10.3390/pharmaceutics13111863. Pharmaceutics. 2021. PMID: 34834277 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
