Long-Term Infectious and Noninfectious Outcomes of Monthly Alemtuzumab as a Calcineurin Inhibitor- and Steroid-Free Regimen for Pancreas Transplant Recipients
- PMID: 33101558
- PMCID: PMC7569440
- DOI: 10.1155/2020/8883183
Long-Term Infectious and Noninfectious Outcomes of Monthly Alemtuzumab as a Calcineurin Inhibitor- and Steroid-Free Regimen for Pancreas Transplant Recipients
Abstract
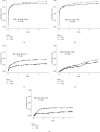
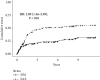
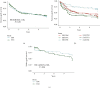
Multiple doses of alemtuzumab for immunosuppressive therapy of patients with hematologic malignancies and hematopoietic stem cell transplant have been associated with a high rate of infection. In transplantation, limited alemtuzumab dosing has been successfully used as induction immunosuppression. The effect of multiple doses of alemtuzumab, used as maintenance therapy to minimize steroid and/or calcineurin inhibitor toxicity in solid organ transplant recipients, is unknown. We evaluated the infectious and noninfectious outcomes of 179 pancreas transplant recipients treated with alemtuzumab for induction and maintenance therapy (extended alemtuzumab exposure (EAE)) from 2/28/2003 through 8/31/2005, compared with 159 pancreas transplant recipients with standard induction and maintenance (SIM) therapy performed before (1/1/2002 until 12/31/2002) and after (1/1/2006 until 12/31/2006) the implementation of EAE. EAE was associated with higher risk of overall infections (hazard ratio (HR) 1.33 (1.06-1.66), P=0.01), bacterial infections (HR 1.33 (1.05-1.67), P=0.02), fungal infections (HR 1.86 (1.28-2.71), P < 0.01), and cytomegalovirus infections (HR 2.29 (1.39-3.77), P < 0.01). In addition, EAE was associated with higher risk of acute cellular rejection (HR 2.09 (1.46-2.99), P < 0.01). In conclusion, while a limited alemtuzumab dosing is safe and effective for induction therapy in pancreas transplantation, EAE combined with steroid and calcineurin minimization is associated with a high risk of infectious complications and acute cellular rejection.
Copyright © 2020 Adam Kaplan et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- Bertz H., Spyridonidis A., Wasch R., Grullich C., Egger M., Finke J. A novel GVHD-prophylaxis with low-dose alemtuzumab in allogeneic sibling or unrelated donor hematopoetic cell transplantation: the feasibility of deescalation. Biology of Blood and Marrow Transplantation. 2009;15(12):1563–1570. doi: 10.1016/j.bbmt.2009.08.002. - DOI - PubMed
-
- Busemann C., Neumann T., Schulze M., et al. Low-dose alemtuzumab vs. standard policy for prevention of graft-versus-host disease in unrelated and related allogeneic stem cell transplantation-a matched pair analysis. Annals of Hematology. 2013;92(7):945–952. doi: 10.1007/s00277-013-1714-4. - DOI - PubMed
LinkOut - more resources
Full Text Sources

