COPD smokers who switched to e-cigarettes: health outcomes at 5-year follow up
- PMID: 33101622
- PMCID: PMC7549158
- DOI: 10.1177/2040622320961617
COPD smokers who switched to e-cigarettes: health outcomes at 5-year follow up
Abstract
Background and aims: The long-term health effects of the use of electronic cigarettes (ECs) in patients with chronic obstructive pulmonary disease (COPD) are largely unexplored. We present findings from a 5-year prospective assessment of respiratory parameters in a cohort of COPD patients who substantially reduced conventional smoking or achieved abstinence by switching to ECs.
Methods: Patients were evaluated prospectively for their measurements of respiratory exacerbations, spirometric indices, quality of life using the COPD assessment tool (CAT), 6-min walk distance (6MWD), as well as conventional cigarette consumption. Baseline measurements prior to switching to EC use were compared with follow-up visits at 12-, 24-, 48- and 60-months. Age- and sex-matched COPD patients reporting to be regular smokers (not using ECs) were the reference group for the analysis.
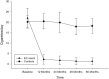
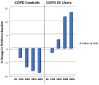
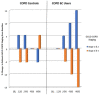
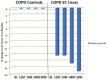
Results: Complete data were available from 39 patients. Those in the EC user group achieved a marked decline in cigarette smoking or abstinence. COPD EC users had a significant diminution in COPD exacerbations; with the mean (±SD) exacerbation rate falling from 2.3 (±0.9) at baseline to 1.1 (±1.0) at 5 years (p < 0.001), whereas no significant changes were observed in the control group.Significant and constant improvements in lung function, CAT scores and 6MWD were reported in the EC user group over the 5-year observation period compared with the reference group (p < 0.05).
Conclusion: The present study suggests that EC use may ameliorate objective and subjective COPD outcomes, and that the benefits gained appear to persist long term. EC use for abstinence and smoking reduction may ameliorate some of the harm resulting from tobacco smoking in COPD patients.
Keywords: COPD; electronic cigarette; smoking cessation; tobacco harm reduction.
© The Author(s), 2020.
Conflict of interest statement
Conflict of interest statement: In relation to his work in the area of tobacco control and respiratory diseases, RP has received lecture fees and research funding from Pfizer, GlaxoSmithKline, CV Therapeutics, NeuroSearch A/S, Sandoz, MSD, Boehringer Ingelheim, Novartis, Duska Therapeutics and Forest Laboratories. He has also served as a consultant for Pfizer, Global Health Alliance for treatment of tobacco dependence, CV Therapeutics, Boehringer Ingelheim, Novartis, Duska Therapeutics, ECITA (Electronic Cigarette Industry Trade Association, in the UK), Arbi Group Srl. and Health Diplomats. RP has been the Director of the Center of Excellence for the acceleration of Harm Reduction at the University of Catania (CoEHAR), which has received a grant from Foundation for a Smoke Free World to develop and carry out eight research projects. RP is also currently involved in the following pro bono activities: scientific advisor for LIAF (Lega Italiana Anti Fumo – Italian acronym for Italian Anti-Smoking League), the Consumer Advocates for Smoke-free Alternatives (CASAA) and the International Network of Nicotine Consumers Organizations (INNCO); Chair of the European Technical Committee for standardization on ‘Requirements and test methods for emissions of electronic cigarettes’ (CEN/TC 437; WG4). All other authors have no relevant conflict of interest to declare in relation to this study. The Associate Editor of Therapeutic Advances in Chronic Disease is an author of this paper, therefore, the peer review process was managed by alternative members of the Board and the submitting Editor had no involvement in the decision-making process
Figures

Similar articles
-
Health effects in COPD smokers who switch to electronic cigarettes: a retrospective-prospective 3-year follow-up.Int J Chron Obstruct Pulmon Dis. 2018 Aug 22;13:2533-2542. doi: 10.2147/COPD.S161138. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 30197510 Free PMC article.
-
Evidence for harm reduction in COPD smokers who switch to electronic cigarettes.Respir Res. 2016 Dec 16;17(1):166. doi: 10.1186/s12931-016-0481-x. Respir Res. 2016. PMID: 27986085 Free PMC article.
-
Health outcomes in COPD smokers using heated tobacco products: a 3-year follow-up.Intern Emerg Med. 2021 Apr;16(3):687-696. doi: 10.1007/s11739-021-02674-3. Epub 2021 Mar 23. Intern Emerg Med. 2021. PMID: 33754228 Free PMC article.
-
E-cigarettes in patients with COPD: current perspectives.Int J Chron Obstruct Pulmon Dis. 2017 Nov 1;12:3203-3210. doi: 10.2147/COPD.S135323. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 29138548 Free PMC article. Review.
-
Health impact of e-cigarettes and heated tobacco products in chronic obstructive pulmonary disease: current and emerging evidence.Expert Rev Respir Med. 2022 Nov-Dec;16(11-12):1213-1226. doi: 10.1080/17476348.2023.2167716. Epub 2023 Jan 22. Expert Rev Respir Med. 2022. PMID: 36638185 Review.
Cited by
-
Improved aerobic capacity in a randomized controlled trial of noncombustible nicotine and tobacco products.Sci Rep. 2025 May 31;15(1):19104. doi: 10.1038/s41598-025-03904-w. Sci Rep. 2025. PMID: 40447739 Free PMC article. Clinical Trial.
-
Smoking cessation program preferences of individuals with chronic obstructive pulmonary disease: a qualitative study.Prim Health Care Res Dev. 2024 Sep 20;25:e38. doi: 10.1017/S1463423624000306. Prim Health Care Res Dev. 2024. PMID: 39301597 Free PMC article.
-
Racial and ethnic disparities in biomarkers of exposure and potential harm among U.S. adult exclusive e-cigarette users: 2013-2019.Drug Alcohol Depend. 2023 Nov 1;252:110984. doi: 10.1016/j.drugalcdep.2023.110984. Epub 2023 Sep 28. Drug Alcohol Depend. 2023. PMID: 37804563 Free PMC article.
-
Association of tobacco product use with chronic obstructive pulmonary disease (COPD) prevalence and incidence in Waves 1 through 5 (2013-2019) of the Population Assessment of Tobacco and Health (PATH) Study.Respir Res. 2022 Oct 1;23(1):273. doi: 10.1186/s12931-022-02197-1. Respir Res. 2022. PMID: 36183112 Free PMC article.
-
Dual Use of Electronic Cigarettes and Cigarettes Elevates Risk of Chronic Obstructive Pulmonary Disease and Mental Health Issues: Insights from a Korean Health Survey.Int J Chron Obstruct Pulmon Dis. 2025 Jun 17;20:1973-1981. doi: 10.2147/COPD.S524978. eCollection 2025. Int J Chron Obstruct Pulmon Dis. 2025. PMID: 40547288 Free PMC article.
References
-
- World Health Organization. WHO report on the global tobacco epidemic 2017. World Health Organization, 2017.
-
- U.S. Department of Health and Human Services. The health consequences of smoking: 50 years of progress: a report of the sugeon general. Atlanta, GA: US Department of Health and Human Services, Centres for Disease Control and Prevention, National Centre for Chronic Disease Prevetnion and Health Promotion, Office on Smoking and Health, 2014.
-
- Morjaria JB, Malerba M, Polosa R. Biologic and pharmacologic therapies in clinical development for the inflammatory response in COPD. Drug Discov Today 2010; 15: 396–405. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

