Alterations of the Human Gut Microbiome in Chronic Kidney Disease
- PMID: 33101877
- PMCID: PMC7578882
- DOI: 10.1002/advs.202001936
Alterations of the Human Gut Microbiome in Chronic Kidney Disease
Abstract
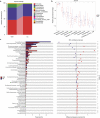
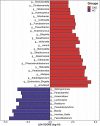
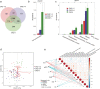
Gut microbiota make up the largest microecosystem in the human body and are closely related to chronic metabolic diseases. Herein, 520 fecal samples are collected from different regions of China, the gut microbiome in chronic kidney disease (CKD) is characterized, and CKD classifiers based on microbial markers are constructed. Compared with healthy controls (HC, n = 210), gut microbial diversity is significantly decreased in CKD (n = 110), and the microbial community is remarkably distinguished from HC. Genera Klebsiella and Enterobacteriaceae are enriched, while Blautia and Roseburia are reduced in CKD. Fifty predicted microbial functions including tryptophan and phenylalanine metabolisms increase, while 36 functions including arginine and proline metabolisms decrease in CKD. Notably, five optimal microbial markers are identified using the random forest model. The area under the curve (AUC) reaches 0.9887 in the discovery cohort and 0.9512 in the validation cohort (49 CKD vs 63 HC). Importantly, the AUC reaches 0.8986 in the extra diagnosis cohort from Hangzhou. Moreover, Thalassospira and Akkermansia are increased with CKD progression. Thirteen operational taxonomy units are correlated with six clinical indicators of CKD. In conclusion, this study comprehensively characterizes gut microbiome in non-dialysis CKD and demonstrates the potential of microbial markers as non-invasive diagnostic tools for CKD in different regions of China.
Keywords: chronic kidney disease; gut microbiome; microbial markers; non‐invasive diagnostic tools.
© 2020 The Authors. Published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
