Effectiveness a herbal medicine (Sipjeondaebo-tang) on adults with chronic fatigue syndrome: A randomized, double-blind, placebo-controlled trial
- PMID: 33101925
- PMCID: PMC7578262
- DOI: 10.1016/j.imr.2020.100664
Effectiveness a herbal medicine (Sipjeondaebo-tang) on adults with chronic fatigue syndrome: A randomized, double-blind, placebo-controlled trial
Abstract
Background: Sipjeondaebo-tang (SJDBT, Shi-quan-da-bu-tang in Chinese) is a widely prescribed herbal medicine in traditional Korean medicine. This study aimed to evaluate the effectiveness and safety of SJDBT for treating chronic fatigue syndrome (CFS).
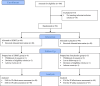
Methods: Ninety-six eligible participants were randomly allocated to either the SJDBT or placebo groups in a 1:1 ratio. Nine grams of SJDBT or placebo granules were administered to the patients for 8 weeks. The primary outcome was the response rate, defined as the proportion of participants with a score of 76 or higher in the Checklist Individual Strength assessment. Other measurements for fatigue severity, quality of life, and qi/blood/yin/yang deficiency were included. Safety was assessed throughout the trial.
Results: At week 8, the response rate did not significantly differ between the groups (SJDBT: 35.4%; placebo: 54.2%; P = 0.101, effect size [95% confidence interval] = 0.021 [-0.177, 0.218]). However, the scores of the visual analogue scale (P = 0.001, -0.327 [-0.506, -0.128]), Fatigue Severity Scale (P = 0.020, 0.480 [0.066, 0.889]), and Chalder fatigue scale (P = 0.004, -0.292 [-0.479, -0.101]) for the SJDBT group showed significant improvements in fatigue severity at the endpoint. Quality of life was not significantly different. Furthermore, SJDBT significantly ameliorated the severity of qi deficiency compared to that in the placebo group. No serious adverse events were observed.
Conclusion: This trial failed to show a significant improvement in fatigue severity, as assessed by the CIS-deprived response rate. It merely showed that SJDBT could alleviate the severity of fatigue and qi deficiency in patients with CFS. However, the further study is needed to confirm the details.
Keywords: Chronic fatigue syndrome; Complementary therapies; Herbal medicine; Randomized controlled trial.
© 2020 Korea Institute of Oriental Medicine. Publishing services by Elsevier B.V.
Figures
Similar articles
-
Traditional Herbal Medicine, Sipjeondaebo-Tang, for Cancer-Related Fatigue: A Randomized, Placebo-Controlled, Preliminary Study.Integr Cancer Ther. 2021 Jan-Dec;20:15347354211040830. doi: 10.1177/15347354211040830. Integr Cancer Ther. 2021. PMID: 34672230 Free PMC article. Clinical Trial.
-
Sipjeondaebo-tang in patients with breast cancer with fatigue: a protocol for a pilot, randomised, double-blind, placebo-controlled, cross-over trial.BMJ Open. 2018 Jul 6;8(7):e021242. doi: 10.1136/bmjopen-2017-021242. BMJ Open. 2018. PMID: 29982213 Free PMC article.
-
Sijunzi decoction, a classical Chinese herbal formula, improves fatigue symptoms with changes in gut microbiota in chronic fatigue syndrome: A randomized, double-blind, placebo-controlled, multi-center clinical trial.Phytomedicine. 2024 Jul;129:155636. doi: 10.1016/j.phymed.2024.155636. Epub 2024 Apr 13. Phytomedicine. 2024. PMID: 38640860 Clinical Trial.
-
Efficacy and Safety of Sipjeondaebo-Tang for Anorexia in Patients with Cancer: A Pilot, Randomized, Double-Blind, Placebo-Controlled Trial.Evid Based Complement Alternat Med. 2017;2017:8780325. doi: 10.1155/2017/8780325. Epub 2017 Dec 26. Evid Based Complement Alternat Med. 2017. PMID: 29441116 Free PMC article.
-
[Effects of Xiaopi Yishen herbal extract granules in treatment of fatigue-predominant subhealth due to liver-qi stagnation and spleen-qi deficiency: a prospective, randomized, placebo-controlled and double-blind clinical trial].Zhong Xi Yi Jie He Xue Bao. 2011 May;9(5):515-24. doi: 10.3736/jcim20110508. Zhong Xi Yi Jie He Xue Bao. 2011. PMID: 21565137 Clinical Trial. Chinese.
Cited by
-
Effectiveness of Chinese Herbal Medicine in Postoperative Fatigue Syndrome Following Total Joint Arthroplasty or Hip Fracture Surgery: Evidence from Randomized Controlled Trials.Comb Chem High Throughput Screen. 2024;27(15):2206-2215. doi: 10.2174/0113862073258802231107060433. Comb Chem High Throughput Screen. 2024. PMID: 38031783 Free PMC article.
-
Traditional Herbal Medicine, Sipjeondaebo-Tang, for Cancer-Related Fatigue: A Randomized, Placebo-Controlled, Preliminary Study.Integr Cancer Ther. 2021 Jan-Dec;20:15347354211040830. doi: 10.1177/15347354211040830. Integr Cancer Ther. 2021. PMID: 34672230 Free PMC article. Clinical Trial.
-
Recent research in myalgic encephalomyelitis/chronic fatigue syndrome: an evidence map.Health Technol Assess. 2025 Mar 26:1-78. doi: 10.3310/BTBD8846. Online ahead of print. Health Technol Assess. 2025. PMID: 40162526 Free PMC article.
-
Chinese herbal medicine for the treatment of chronic fatigue syndrome: A systematic review and meta-analysis.Front Pharmacol. 2022 Sep 29;13:958005. doi: 10.3389/fphar.2022.958005. eCollection 2022. Front Pharmacol. 2022. PMID: 36249791 Free PMC article.
-
Ginsenoside Rg1 can reverse fatigue behavior in CFS rats by regulating EGFR and affecting Taurine and Mannose 6-phosphate metabolism.Front Pharmacol. 2023 Apr 10;14:1163638. doi: 10.3389/fphar.2023.1163638. eCollection 2023. Front Pharmacol. 2023. PMID: 37101547 Free PMC article.
References
-
- Fukuda K., Straus S.E., Hickie I., Sharpe M.C., Dobbins J.G., Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources