Evaluation of acute and sub-acute toxicity of selected traditional antiurolithiatic medicinal plant extracts in Wistar albino rats
- PMID: 33102139
- PMCID: PMC7569265
- DOI: 10.1016/j.toxrep.2020.10.001
Evaluation of acute and sub-acute toxicity of selected traditional antiurolithiatic medicinal plant extracts in Wistar albino rats
Abstract
Introduction: Achyranthes aspera, Chenopodium murale, Satureja punctata, Rumex abyssinicus and Aloe pulcherrima are traditionally used to treat urolithiasis in Ethiopia. However, there are limited reports on toxicity studies.
Objective: This study was intended to evaluate the acute and sub-acute toxicity effects of plants.
Materials and methods: The crude extracts of A. aspera and C. murale leaves, S. punctata aerial parts, R. abyssinicus rhizomes, and A. Pulcherrima gel were prepared using 70 % ethanol. In acute toxicity, 125, 500 and 2000 mg/kg were tested in a stepwise manner; whereas 2000 mg/kg administrated to female rats using gavage during sub-acute toxicity. On day 14 and 28, blood samples were collected from retro-orbital sinus; liver and kidneys of each animal were collected under anaesthesia. Data were analyzed using one-way ANOVA, Dunnett's comparison test of the Graph Pad Prism.
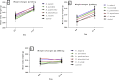
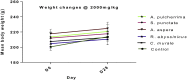
Results: No mortality and significant weight loss for all extracts in both toxicity tests. In acute toxicity, C. murale extract significantly reduced hemoglobin and platelets (P < 0.01) compared with the control. Likewise, S. punctata (P < 0.05) and R. abyssinicus (P < 0.01) extracts revealed significant reduction in platelet count. An exposure to C. murale and R. abyssinicus extracts reduced the concentrations of platelet distribution width and platelet larger cell ratio (p < 0.05) during sub-acute toxicity test. The level of creatinine reduced due to A. aspera extract administrations(P < 0.05). Liver histopathological examinations revealed focal periportal hepatitis following sub-acute toxicity test of C. murale. Histopathological studies of liver demonstrated that R. abyssinicus, A. aspera and S. punctata extracts showed mild acute liver injury. A. pulcherrima was not associated with any toxicity.
Conclusion: C. murale extract showed hematological, and histopathological toxicity profiles in rats. Furthermore, chronic toxicity studies of A. aspera, S. punctata and R. abyssinicus extracts would be beneficial to ensure safety.
Keywords: Acute toxicity; Albino wistar female rats; Antiurolithiatic plant extracts; Sub-acute toxicity.
© 2020 Published by Elsevier B.V.
Conflict of interest statement
The authors report no declarations of interest.
Figures





References
-
- Ankur C., Amarchand P., Aadarsh C., Deepa I., Pawar R.S., Patil U.K. Potential of medicinal plants in kidney, gall and urinary stones. Int. J. Drug Dev. Res. 2010;2(2):431–447.
-
- Diallo D., Paulsen B.S., Hvemm B. Production of traditional medicine: preparations accepted as medicines in Mali. In: Hostettmann K., Chinyanganya F., Maillard M., Wolfender J.L., editors. Chemistry, Biological and Pharmacological Properties of African Medicinal Plants: Proceedings of the First International IOCD-Symposium. UZ Publications; Victoria Falls, Zimbabwe: 1996. pp. 235–243. February 25-28 Harare.
-
- Endashaw B. Study on actual situation of medicinal plants in Ethiopia. Japan Assoc. Int.Coll. Agric. Fores. 2007 http://www.jaicaf.or.jp/publications/ethiopiaac.pdf
-
- Rates S.M. Plants as source of drugs. Toxicon. 2001;39(5):603–613. - PubMed
LinkOut - more resources
Full Text Sources

