Virulence Regulation and Innate Host Response in the Pathogenicity of Vibrio cholerae
- PMID: 33102256
- PMCID: PMC7554612
- DOI: 10.3389/fcimb.2020.572096
Virulence Regulation and Innate Host Response in the Pathogenicity of Vibrio cholerae
Abstract
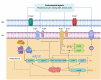
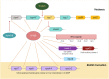
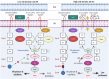
The human pathogen Vibrio cholerae is the causative agent of severe diarrheal disease known as cholera. Of the more than 200 "O" serogroups of this pathogen, O1 and O139 cause cholera outbreaks and epidemics. The rest of the serogroups, collectively known as non-O1/non-O139 cause sporadic moderate or mild diarrhea and also systemic infections. Pathogenic V. cholerae circulates between nutrient-rich human gut and nutrient-deprived aquatic environment. As an autochthonous bacterium in the environment and as a human pathogen, V. cholerae maintains its survival and proliferation in these two niches. Growth in the gastrointestinal tract involves expression of several genes that provide bacterial resistance against host factors. An intricate regulatory program involving extracellular signaling inputs is also controlling this function. On the other hand, the ability to store carbon as glycogen facilitates bacterial fitness in the aquatic environment. To initiate the infection, V. cholerae must colonize the small intestine after successfully passing through the acid barrier in the stomach and survive in the presence of bile and antimicrobial peptides in the intestinal lumen and mucus, respectively. In V. cholerae, virulence is a multilocus phenomenon with a large functionally associated network. More than 200 proteins have been identified that are functionally linked to the virulence-associated genes of the pathogen. Several of these genes have a role to play in virulence and/or in functions that have importance in the human host or the environment. A total of 524 genes are differentially expressed in classical and El Tor strains, the two biotypes of V. cholerae serogroup O1. Within the host, many immune and biological factors are able to induce genes that are responsible for survival, colonization, and virulence. The innate host immune response to V. cholerae infection includes activation of several immune protein complexes, receptor-mediated signaling pathways, and other bactericidal proteins. This article presents an overview of regulation of important virulence factors in V. cholerae and host response in the context of pathogenesis.
Keywords: V. cholerae; host response; microbiome; quorum sensing; toxins; virulence.
Copyright © 2020 Ramamurthy, Nandy, Mukhopadhyay, Dutta, Mutreja, Okamoto, Miyoshi, Nair and Ghosh.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

