Emerging Evasion Mechanisms of Macrophage Defenses by Pathogenic Bacteria
- PMID: 33102257
- PMCID: PMC7545029
- DOI: 10.3389/fcimb.2020.577559
Emerging Evasion Mechanisms of Macrophage Defenses by Pathogenic Bacteria
Abstract
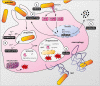
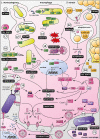
Macrophages participate to the first line of defense against infectious agents. Microbial pathogens evolved sophisticated mechanisms to escape macrophage killing. Here, we review recent discoveries and emerging concepts on bacterial molecular strategies to subvert macrophage immune responses. We focus on the expanding number of fascinating subversive tools developed by Listeria monocytogenes, Staphylococcus aureus, and pathogenic Yersinia spp., illustrating diversity and commonality in mechanisms used by microorganisms with different pathogenic lifestyles.
Keywords: immune escape; listeriosis; phagocyte; plague; staphylococcal infection; virulence; yersiniosis.
Copyright © 2020 Leseigneur, Lê-Bury, Pizarro-Cerdá and Dussurget.
Figures
References
-
- Banerjee S. K., Huckuntod S. D., Mills S. D., Kurten R. C., Pechous R. D. (2019). Modeling pneumonic plague in human precision-cut lung slices highlights a role for the plasminogen activator protease in facilitating type 3 secretion. Infect. Immun. 87, e00175–e00119. 10.1128/IAI.00175-19 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

