Stability, Aromaticity, and Photophysical Behaviors of Macrocyclic Molecules: A Theoretical Analysis
- PMID: 33102432
- PMCID: PMC7500243
- DOI: 10.3389/fchem.2020.00776
Stability, Aromaticity, and Photophysical Behaviors of Macrocyclic Molecules: A Theoretical Analysis
Abstract
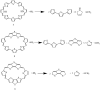
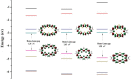
The macrocyclic molecules with terthiophene (TTH) isomers unit exhibit intriguing properties in terms of aromaticity, stability, and absorption. In this work, we theoretically designed a series of macrocyclic molecules featured with TTH and dithienothiophene (DTT) π-conjugated building units, which are used to permute pyrrole unit in porphyrin skeleton. Density functional theory and time-dependent DFT methods are used to evaluate the performance of the designed molecules. Our simulations show that molecules 1-3 exhibit excellent optoelectronic performance. Specifically, the molecule with the DTT unit is more stable than the one with TTH unit in terms of aromaticity and aromatic stabilization energy. This is because DTT unit enhances the coplanarity of the molecular material, facilitating electronic communication. Calculation of vertical electronic excitations suggests the absorption feature of these molecules is mainly contributed by the electronic excitations of higher occupied molecular orbital (HOMO) → lowest unoccupied molecular orbital (LUMO)+1 and HOMO-1 → LUMO. Judging from the key parameters determining the overall performance, 3 stands out because of its good planarity, large HOMO-LUMO gap, and strong aromaticity among all molecules. Interestingly, molecule 1 has the current density flow distributes around the outer section of TTH unit; in contrast, molecule 3 with DTT unit has the current density flow located at the inner section of DTT, which is beneficial for stability and aromaticity. Second-order perturbation energies are calculated to rationalize this observation. We expect that these research results can provide valuable insights into the rational design of novel molecular materials for a variety of applications.
Keywords: DFT/TD-DTF; aromaticity; macrocyclic molecules; molecular modification; porphyrin.
Copyright © 2020 Wei, Ren, Jian, Xia, Zhang, Bai and Li.
Figures



References
-
- Aihara J. I. (1999). Reduced HOMO–LUMO gap as an index of kinetic stability for polycyclic aromatic hydrocarbons. J. Phys. Chem. A 103, 7487–7495. 10.1021/jp990092i - DOI
-
- Akaishi A., Ushirozako M., Matsuyama H., Nakamura J. (2018). Structural stability and aromaticity of pristine and doped graphene nanoflakes. Jpn. J. Appl. Phys. 57, 0102BA 1–8. 10.7567/JJAP.57.0102BA - DOI
-
- Bader R. F. W., Popelier P. L. A., Keith T. A. (1994). Theoretical definition of a functional group and the molecular orbital paradigm. Angew. Chem. Int. Ed. 33, 620–631. 10.1002/anie.199406201 - DOI
-
- Broadhurst M. J., Grigg R., Johnson A. W. (1972). The synthesis of 22 π-electron macrocycles. Sapphyrins and related compounds. J. Chem. Soc. Perkin Trans. 1, 2111–2116. 10.1039/P19720002111 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

