Energy Landscape for the Membrane Fusion Pathway in Influenza A Hemagglutinin From Discrete Path Sampling
- PMID: 33102445
- PMCID: PMC7546250
- DOI: 10.3389/fchem.2020.575195
Energy Landscape for the Membrane Fusion Pathway in Influenza A Hemagglutinin From Discrete Path Sampling
Abstract
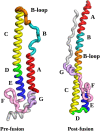
The conformational change associated with membrane fusion for Influenza A Hemagglutinin is investigated with a model based upon pre- and post-fusion structures of the HA2 component. We employ computational methods based on the potential energy landscape framework to obtain an initial path connecting these two end points, which provides the starting point for refinement of a kinetic transition network. Here we employ discrete path sampling, which provides access to the experimental time and length scales via geometry optimization techniques to identify local minima and the transition states that connect them. We then analyse the distinct phases of the predicted pathway in terms of structure and energetics, and compare with available experimental data and previous simulations. Our results provide the foundations for future work, which will address the effect of mutations, changes in pH, and incorporation of additional components, especially the HA1 chain and the fusion peptide.
Keywords: discrete path sampling; energy landscape; influenza; membrane fusion; rare event algorithms.
Copyright © 2020 Burke, Mantell, Pitt and Wales.
Figures

References
-
- Becker O. M., Karplus M. (1997). The topology of multidimensional potential energy surfaces: theory and application to peptide structure and kinetics. J. Chem. Phys. 106:1495 10.1063/1.473299 - DOI
-
- Broyden C. G. (1970). The convergence of a class of double-rank minimization algorithms 1. general considerations. J. Inst. Math. Appl. 6, 76–90. 10.1093/imamat/6.1.76 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

