Production of the Fragrance Geraniol in Peroxisomes of a Product-Tolerant Baker's Yeast
- PMID: 33102464
- PMCID: PMC7546902
- DOI: 10.3389/fbioe.2020.582052
Production of the Fragrance Geraniol in Peroxisomes of a Product-Tolerant Baker's Yeast
Abstract
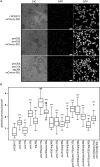
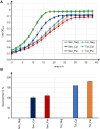
Monoterpenoids, such as the plant metabolite geraniol, are of high industrial relevance since they are important fragrance materials for perfumes, cosmetics, and household products. Chemical synthesis or extraction from plant material for industry purposes are complex, environmentally harmful or expensive and depend on seasonal variations. Heterologous microbial production offers a cost-efficient and sustainable alternative but suffers from low metabolic flux of the precursors and toxicity of the monoterpenoid to the cells. In this study, we evaluated two approaches to counteract both issues by compartmentalizing the biosynthetic enzymes for geraniol to the peroxisomes of Saccharomyces cerevisiae as production sites and by improving the geraniol tolerance of the yeast cells. The combination of both approaches led to an 80% increase in the geraniol titers. In the future, the inclusion of product tolerance and peroxisomal compartmentalization into the general chassis engineering toolbox for monoterpenoids or other host-damaging, industrially relevant metabolites may lead to an efficient, low-cost, and eco-friendly microbial production for industrial purposes.
Keywords: Saccharomyces cerevisiae; compartmentalization; geraniol; monoterpenoids; peroxisomes; tolerance.
Copyright © 2020 Gerke, Frauendorf, Schneider, Wintergoller, Hofmeister, Poehlein, Zebec, Takano, Scrutton and Braus.
Figures



References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

