Additive Manufacturing of Nerve Guidance Conduits for Regeneration of Injured Peripheral Nerves
- PMID: 33102468
- PMCID: PMC7546374
- DOI: 10.3389/fbioe.2020.590596
Additive Manufacturing of Nerve Guidance Conduits for Regeneration of Injured Peripheral Nerves
Abstract
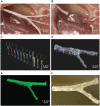
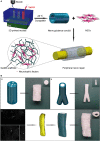
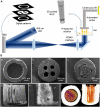
As a common and frequent clinical disease, peripheral nerve defect has caused a serious social burden, which is characterized by poor curative effect, long course of treatment and high cost. Nerve autografting is first-line treatment of peripheral nerve injuries (PNIs) but can result in loss of function of the donor site, neuroma formation, and prolonged operative time. Nerve guidance conduit (NGC) serves as the most promising alternative to autologous transplantation, but its production process is complicated and it is difficult to effectively combine growth factors and bioactive substances. In recent years, additive manufacturing of NGCs has effectively solved the above problems due to its simple and efficient manufacturing method, and it can be used as the carrier of bioactive substances. This review examines recent advances in additive manufacture of NGCs for PNIs as well as insight into how these approaches could be improved in future studies.
Keywords: additive manufacturing; biomaterial; nerve guidance conduit; nerve regeneration; peripheral nerve.
Copyright © 2020 Song, Wang, Wang, Yu, Hou, Zhu and Li.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

